Consider the following decomposition reaction in which 47.20 g of some compound is decomposed into its elements.
Question:
Consider the following decomposition reaction in which 47.20 g of some compound is decomposed into its elements.
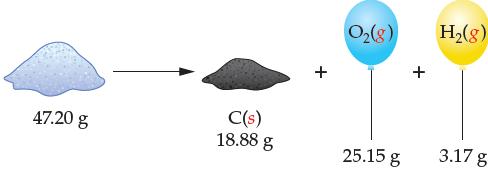
(a) What is the percent composition of each
element in the original compound?
(b) What is the empirical formula of the compound?
(c) The molar mass of the compound is
60.05 g/mol. What is the actual formula of the
compound?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





