Consider the two nonpolar hydrocarbon molecules, both of formula C 5 H 12 . Having exactly the
Question:
Consider the two nonpolar hydrocarbon molecules, both of formula C5H12.
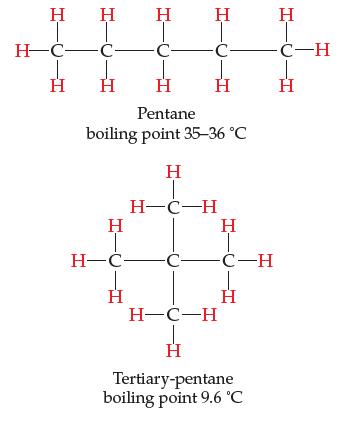
Having exactly the same formula, they have exactly the same number of electrons, yet pentane has a higher boiling point than tertiary-pentane. Discuss why and come up with a theory that explains why.
Transcribed Image Text:
Η Η H¬C-C- Ι H Η Η HIC Η Η -C- Η Η Pentane boiling point 35–36 °C H-C- Η Η H-C-H C- 1-0- Η -C-H Η H-C-H Η Tertiary-pentane boiling point 9.6 °C Η -C-H Η
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Pentane and tertiarypentane also called neopentane have the same molecular formula C5H12 but they ha...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Suppose the relationship between wage, years of education (educ), years of experience (exper), and participation in a job training program (train) is modeled as: log ( ) = + + + . Which of the...
-
Explain why a. H2O has a higher boiling point than CH3OH(65oC). b. H2O has a higher boiling point than NH3(- 33oC). c. H2O has a higher boiling point than HF (20C).
-
The symmetrical three-phase load is A-connected, with Z-(4+ j3) 22 and the phase voltage 220 V, find IL, IP, UL and P of the three loads. 7.(10 score) The symmetrical three-phase load is Y-connected....
-
Youre in charge of pricing Sonics product for its launch early next year.Review the SWOT analysis you previously prepared as well as Sonics competitive environment, targeting strategy, and product...
-
Linda Kipp started a business on May 1, 20--. Analyze the following transactions for the first month of business using T accounts. Label each T account with the title of the account affected and then...
-
1. Identify 3 students to play the roles of the employees. Ask these 3 individuals to read their roles below. 2. Identify 1 student to play the role of the president of the social enterprise (Taylor...
-
Lager Dental Clinic is a medium-sized dental service specializing in family dental care. The clinic is currently preparing the master budget for the first 2 quarters of 2014. All that remains in this...
-
Research Web-based database technologies and identify a database management system (other than SQL Server, MySQL, or Oracle) that is used to deploy applications to the Web and the cloud. Discuss the...
-
Based on the strength of their molecular dipole moments, which compound should have the higher boiling point, HF or HBr? Explain.
-
A condensed phase is (a) A shortened (condensed) version of a substance. (b) The gas form of a substance. (c) The liquid and solid forms of a substance. (d) Formed by evaporation.
-
Which statement best describes the results of Exercise 32 ? i. For each of the four soils, we are \(95 \%\) confident that the mean speed is greater when the torque is lower. ii. For each of the four...
-
B Marcovich pays cash for three vehicles on 2 January 2023 for $38 500 ($35 000 + $3500 GST) each. Motor vehicle 1 will be replaced after five years and has an estimated residual of $2200 ($2000 +...
-
R Gilmour & Associates purchased and installed on credit a computer system that was commissioned on 1 April 2021 for $66 000 ($60 000 + $6000 GST). It is expected that the computer system has a...
-
The accounts of P L Lorikeet revealed a number of matters that require attention and correction by general journal. a Cash receipts journal totals for the month were posted to the general ledger only...
-
Entcomm, located in a small ex-industrial town near Glasgow, provides telecommunications and entertainment services for a large US multinational company. The call centre handles inquiries, billing,...
-
What is the total of entries made to each of the five journals from the following list of source documents? Source Document Tax invoice to customer Remittance received Tax invoice to customer...
-
The following information is available for Mitchelville Products Company for the month of July: Required 1. Set up a spreadsheet to compute the July sales volume variance and the flexible-budget...
-
Suppose a population of bacteria doubles every hour, but that 1.0 x 106 individuals are removed before reproduction to be converted into valuable biological by-products. Suppose the population begins...
-
Draw a bond-line structure for each of the following amino acids. (a) l-Leucine (b) l-Tryptophan (c) l-Methionine (d) l-Valine
-
Although most naturally occurring proteins are made up only of l amino acids, proteins isolated from bacteria will sometimes contain d amino acids. Draw Fischer projections for d-alanine and...
-
Compound A is a d-aldopentose. When treated with sodium borohydride, compound A is converted into an alditol that exhibits three signals in its 13C NMR spectrum. Compound A undergoes a...
-
3. Strategic Choices: What are the main strategic choices available to MIHI in the cannabis market? Discuss the pros and cons of each. o How does MIHI differentiate itself from competitors in the...
-
Describe what anemia is and what causes the various forms of its types such as pernicious, sickle cell among others
-
2. Given the discrete systems below, show on the z-plane the possible regions of convergence. 2.1 G = 2.2 G 2.3 Gz = = 2+1 (23+2 + 2) z 2 +z+1 (z 0.1)(22 +z+ 0.5) - z 2 +z+1 (z 0.1)(z+z+0.5)

Study smarter with the SolutionInn App


