Question: Consider the two square-planar molecules shown below. Which would you expect to have a higher boiling point, and why? F CI-Xe Cl 1-8 F (a)
Consider the two square-planar molecules shown below. Which would you expect to have a higher boiling point, and why?
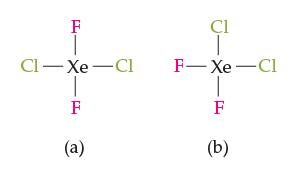
F CI-Xe Cl 1-8 F (a) Cl F-Xe-Cl T F (b)
Step by Step Solution
★★★★★
3.33 Rating (168 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Between the two squareplanar molecules shown a FClXeClF b FXeClF We can predict that molecule a FClX... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


