If we continuously compress a real gas in a cylinder with a movable piston while keeping the
Question:
If we continuously compress a real gas in a cylinder with a movable piston while keeping the container at a constant temperature in an ice bath:
(a) What will eventually happen to the gas and why?
(b) How well will the ideal gas law apply to the real gas shown when it is close to doing what you said it will do in part (a)?
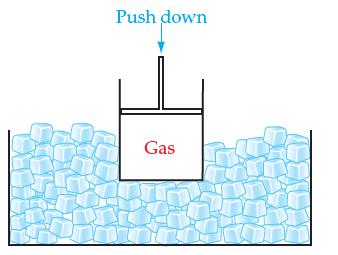
Transcribed Image Text:
Push down Gas
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a If we continuously compress a real gas in a cylinder with a movable piston while keeping the container at a constant temperature in an ice bath the ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
A(n) ________ is a short-lived fashion. Relatively few people adopt this product, but it can spread quickly. Question content area bottom Part 1 A. fad B. creolization C. aspirational reference D....
-
Ivy Enterprises Inc. manufactures bathroom fixtures. The stockholders equity accounts of Ivy Enterprises Inc., with balances on January 1, 2010, are as follows: The following selected transactions...
-
The now defunct Enren Corporation, once headquartered in Houston. Texas, provided produces and services for natural gas, electricity, and communications to wholesale and retail customers. Enrons...
-
a. What is the de Broglie wavelength of a \(200 \mathrm{~g}\) baseball with a speed of \(30 \mathrm{~m} / \mathrm{s}\) ? b. What is the speed of a \(200 \mathrm{~g}\) baseball with a de Broglie...
-
Personal injury lawyers may be paid a contingency fee equal to a percentage of the amount awarded. The lawyer receives payment only if his or her client wins the case and is awarded a sum of money....
-
What ecosystem integrations and compatibility enhancements does MariaDB offer, including compatibility with MySQL APIs, connectors for popular programming languages and frameworks, and...
-
You are trapped on a desert island without any reference books, and you need the value of the ideal gas constant R. Luckily, you stumble upon the following tank of helium (see below). Use what you...
-
Consider two balloons, both in the same room and both exactly the same volume. Consider also the balanced reaction between these gases. If we allow the gases from these balloons to mix and react,...
-
Levon Helm was a kind of one- man mortgage broker. He would drive around Tennessee looking for homes that had second mortgages, and if the criteria were favorable, he would offer to buy the second...
-
Compare how individualistic and collectivistic cultures are oriented, generally, with respect to conflict, according to face-negotiation theory
-
Directions: Analyze each of the following arguments in terms of new case, comparison cases, known similarities, and inferred similarity. Then indicate what seems to you the most significant relevant...
-
Explain why we do each of the first 5 steps in a customer interaction.? Also discuss the 5 steps
-
Compare and contrast two of Aristotle's three types of proofs
-
Find and explain the conventional meaning of 3 symbols in Blake's poem "The Chimney Sweeper". What is the writer's purpose in using these symbols? William Blake "The Chimney Sweeper" Songs of...
-
Use any print or CATR service or the Internet to find a Code section(s) on the following income topics. For each item, indicate the Code section number(s) and full title of the relevant Code...
-
A fuel pump sends gasoline from a car's fuel tank to the engine at a rate of 5.88 10-2 kg/s. The density of the gasoline is 735 kg/m3, and the radius of the fuel line is 3.18 10-3 m. What is the...
-
The circuit in Fig. 2.134 is to control the speed of a motor such that the motor draws currents 5 A, 3 A, and 1 A when the switch is at high, medium, and low positions, respectively. The motor can be...
-
Find R ab in the four-way power divider circuit in Fig. 2.135 . Assume each R = 4 . ww- ww- ER ER R. :R
-
Repeat Prob. 2.75 for the eight-way divider shown in Fig. 2.136 . Prob 2.75 Find R ab in the four-way power divider circuit in Fig. 2.135. Assume each R = 4 Ω. inIim LinLw Lui bo ww- ww-...
-
Discuss the role of the cell cycle in stem cell self-renewal, differentiation, and tissue regeneration, including the coordination of cell cycle progression with developmental signaling pathways,...
-
In two pages, In order for products to be successful in global markets, considerable research must be conducted to ensure the product will meet the needs of locals. Marketers must modify the 4P's to...
-
On January 1, 2019, Jerome Company purchased nontrading equity investments which are irrevocably designated at FVOCI Purchase Transaction price Market value December 31, 2019 cost Security A Security...

Study smarter with the SolutionInn App


