Indicate whether each reaction is endothermic or exothermic: (a) CO + 2 HOCH4 +2O AErxn = +890
Question:
Indicate whether each reaction is endothermic or exothermic:
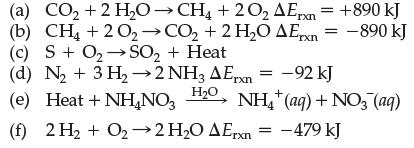
Transcribed Image Text:
(a) CO₂ + 2 H₂OCH4 +2O₂ AErxn = +890 kJ (b) CH4 + 2O₂ →CO₂ + 2 H₂O AErx = -890 kJ (c) S + O₂ SO₂ + Heat (d) N₂+ 3 H₂→2 NH3 AErxn= -92 kJ H₂O (e) Heat + NH₂NO3 (f) 2 H₂ + O₂-2 H₂O AErxn = -479 kJ NH₂(aq) + NO3 (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
All of the reactions in the image are endothermic meaning that they absorb heat from the surrounding...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Determine whether each process is exothermic or endothermic and indicate the sign of H. a. Dry ice evaporating b. A sparkler burning c. The reaction that occurs in a chemical cold pack used to ice...
-
Determine the value of E a and E rxn for each case below. Also indicate whether each reaction is endothermic or exothermic. (a) (b) (c) (d) Energy of reactants, kJ 100 100 50 20 Energy of Energy of...
-
The following diagram shows the Gint coefficient and income share of the B40. M40 and T20 groups in Malaysia between the years 1970 and 2019. Income Share (%) 70.0 60.0 55.7 50.0 40.0 30.0 20.0 10.0...
-
A non reactive/conservative contaminant is dumped on the ground level and it leaches to the groundwater vertically and takes half day for reaching the groundwater by travelling through unsaturated...
-
Finding Financial Information Refer to the financial statements of Urban Outfitters in Appendix C at the end of this book. Required: 1. How much is in the Prepaid Expenses and Other Current Assets...
-
The Dauten Toy Corporation uses an injection molding machine that was purchased 2 years ago. This machine is being depreciated on a straight-line basis, and it has 6 years of remaining life. Its...
-
What are the \(n\) and \(l\) values of the following states of a hydrogen atom: (a) \(4 d\), (b) \(5 f\), (c) \(6 s\) ?
-
A bonding operation utilizes a laser to provide a constant heat flux, q"0, across the top surface of a thin adhesive-backed, plastic film to be affixed to a metal strip as shown in the sketch. The...
-
Kubin Company's relevant range of production is 18,000 to 22,000 units. When it produces and sells 20,000 units, its average costs per unit are as follows: Average Cost per Unit Fixed administrative...
-
Fill in the blanks. When a chemical reaction is in the _______ _______ , the reactant bonds are just ready to break and the product bonds are just ready to form.
-
In each reaction, indicate which bonds are broken and which bonds are formed: (a) N + 3H 2 NH3 (b) PC15 PCl3 + Cl (c) H+2 ICI 2 HCl + 1 (d) 4 HBr + O 2 HO + 2Br2
-
Explain the difference between economic and social value.
-
Do you believe that Lafarge's actions in Syria were ethical or unethical? Use the four methods of ethical reasoning to support your view.
-
Why is a need to chart a positive direction for the future?
-
What is the difference between the American Association for Physical Activity and Recreation (AAPAR) and the National Association for Sport and Physical Education (NASPE)?
-
Consider a job where you are currently employed, or a prior job. A) Perform a job analysis on that job. What tasks are required? What knowledge, skills, and abilities are necessary to perform those...
-
What are some characteristics and risk factors of abusive parents
-
Rikki has the following capital gains and losses for the current year: Short-term capital gain . $ 1,000 Long-term capital gain . 11,000 Long-term capital loss .. 3,000 Collectibles gain .... 8,000...
-
A sprinkler head malfunctions at midfield in an NFL football field. The puddle of water forms a circular pattern around the sprinkler head with a radius in yards that grows as a function of time, in...
-
Deer in the headlights. There are two important time intervals to consider when coming to an emergency stop while driving. The first is the drivers reaction time to get a foot on the brake pedal, and...
-
What is your reaction time? The following simple method can be employed to determine reaction time. A partner holds a meter stick by pinching it at the top and letting it hang vertically. To measure...
-
The kick experienced when firing a rifle can be explained by Newtons third law. A .22-caliber rifle has a mass M = 5.2 kg, and a bullet with a mass m = 3.0 g leaves the barrel of the gun at a...
-
Merrified Company uses the allowance method to account for uncollectible receivables. During 2023, it had total sales of $4,500,000, including $500,000 in cash sales. On December 31, 2023, the...
-
John owns and manages a small business which sells organic beauty products to high street shops. John sells to the shops on credit at 30 days credit terms. (John manages all aspects of the business,...
-
Create a 500 essay on the Prestige performance which is in the last column compared to the industry average of the financial ratio. Please make use of all the ratios with comparable figures and make...

Study smarter with the SolutionInn App


