Some molecules have central atoms with steric numbers greater than 4. For example, the central sulfur atom
Question:
Some molecules have central atoms with steric numbers greater than 4. For example, the central sulfur atom in SF6 has a steric number of 6. Its actual geometry (called octahedral), is shown below (all the bond angles are 90° or 180°).
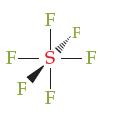
(a) Are the bonds in SF6 polar, polar covalent, or ionic? Explain.
(b) Would you predict the SF6 molecule to be polar or nonpolar? Justify your answer.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





