Suppose the exothermic reaction has come to equilibrium. Which of the following are true? (a) Adding O
Question:
Suppose the exothermic reaction
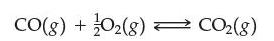
has come to equilibrium. Which of the following are true?
(a) Adding O2(g) will shift the reaction to the left.
(b) Cooling the reaction will shift it to the right.
(c) Cooling the reaction will shift it to the left.
(d) All of the above are true.
Transcribed Image Text:
(8) 10) = (8)' 10 2 OF + CO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
b Cooling ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
15. Mama Corporation (a U.S. taxpayer) has a wholly-owned sales subsidiary in the Bahamas (Bahamamama Ltd.) that purchases finished goods from its U.S. parent and sells those goods to customers...
-
X Finding Values of an Inverse Function Assume that f is a one-to-one function. -25. (a) If f(2)= 7, find f(7). (b) If f(3) = -1, find f(-1). 26. (a) If f(5) = 18, find f(18). (b) If f(4) = 2, find...
-
In the film, Brain Matters, it showed that researchers, scientists and educators have outlined a set of four brain-boosting processes that will strengthen neural pathways and help children to be...
-
McGuire Industries prepares budgets to help manage the company. McGuire is budgeting for the fiscal year ended January 31, 2021. During the preceding year ended January 31, 2020, sales totaled $9,200...
-
Friar Paint Company makes paint in many different colors; it charges the same price for all of its paint regardless of the color. Recently, Friars chief competitor cut the price of its white paint,...
-
The income statement for Jiff y Woodworkers Inc., for the year ended December 31, 2012, is as follows: Dividends of $24,800 were paid on December 30, 2012. Required: 1. Give the entry required on...
-
An inductor is connected to a \(15 \mathrm{kHz}\) oscillator that produces an rms voltage of \(6.0 \mathrm{~V}\). The peak current is \(65 \mathrm{~mA}\). What is the value of the inductance \(L\) ?
-
Best Practices, Inc., is a management consulting firm. Its Corporate Division advises private firms on the adoption and use of cost management systems. Government Division consults with state and...
-
Husky Builders builds manufactured houses on speculation of being able to sell them. Husky uses a normal job costing system and applies overhead on the basis of direct labour hours. The company...
-
Write full nuclear reactions for Practice Problems 16.10 and 16.11. Data from Practice Problems 16.10 The magnesium isotope 25 12 Mg is the daughter isotope created when a radioactive parent isotope...
-
Suppose the shortcut rules can determine the oxidation state of every atom in a compound except one. How can you find the oxidation state of the remaining atom?
-
Consider the corrupt culture at Daimler. Why do you think that the practice of making routine bribes persisted for so many years? Why do you think no one blew the whistle on the practice?
-
Consider the TIC production process through the Toyota production system approach and develop an information solution to support it.
-
Based on your answer to Problem 5-19, if the government aims to correct the positive externality in the inoculation market via a per-unit subsidy to consumers, in the wake of the study, is the...
-
Since the beginning of this century, there has been a significant increase in the price of corn-based ethanol. a. A key input in the production of corn-based ethanol is corn. Use an appropriate...
-
Consider the benefits and limitations of technical standards such as ISO and EFQM in a rapidly evolving digital business environment.
-
(a) Explain the term identifiable, and list assets that would be excluded from intangible assets as a result of this criterion included in the definition of an intangible asset. (b) What is an active...
-
Explain why some people support unions whereas others oppose them.
-
Determine the values of the given trigonometric functions directly on a calculator. The angles are approximate. tan 0.8035
-
Mount McKinley (also called Denali) is the tallest mountain in North America, with a height of 6200 m above sea level. If a person of mass 120 kg walks from sea level to the top of Mount McKinley,...
-
Consider again the roller coaster in Figure P6.32, but now assume the roller coaster starts with a speed of 12 m/s at point A. Find the speed of the roller coaster when it reaches locations B and C....
-
Suppose the ski trail in Problem 31 is not friction less. (a) Find the work done by gravity on the skier in this case. (b) If the skier has a speed of 30 m/s at the bottom of the hill, what is the...
-
Information about Calbee Company is provided below: Ending Accounts Receivable: $ 1 0 0 , 0 0 0 Total Credit Sales: $ 2 5 0 , 0 0 0 Beginning Allowance for Doubtful Accounts: $ 1 5 , 0 0 0 ( Credit...
-
AUCW student is considering a coffee shop business after graduating. 1. The student has limited funds and is considering one retail coffee shop in downtown Vancouver. He assumes he will need at least...
-
Virgin America, a U.S.-based low-fare airline established by the British conglomerate Virgin Group in 2007, was later acquired by Alaska Airlines in 2018. In light of theprovided case article...

Study smarter with the SolutionInn App


