The diagram below shows a ground-state hydrogen atom undergoing excitation to an excited state followed by relaxation.
Question:
The diagram below shows a ground-state hydrogen atom undergoing excitation to an excited state followed by relaxation.
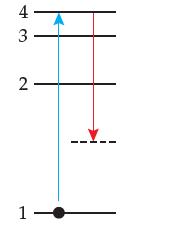
(a) What is fundamentally wrong with the diagram?
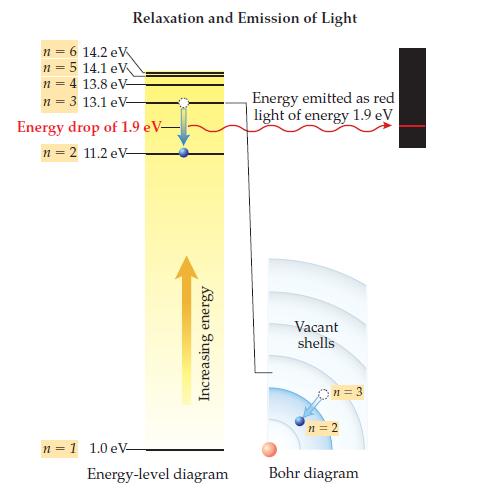
(b) Using the figure on page 133, calculate the amount of energy required to cause the above excitation.
(c) Illustrate at least two allowed relaxations from the excited state shown in the diagram.
Figure from page 133
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





