The equilibrium constant for the reaction (a) Is a solution of ammonium ion very acidic or only
Question:
The equilibrium constant for the reaction
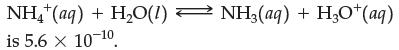
(a) Is a solution of ammonium ion very acidic or only slightly acidic?
(b) Is water acting as an acid or a base according to the Brønsted–Lowry definition? Explain.
Transcribed Image Text:
NH,*(aq) + H,O(1) is 5.6 x 10-10 NH3(aq) + H3O+ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Given the equilibrium constant for the reaction NH4 aq H2O1 NH3aq H3O aq K 56 x 1010 We ca...View the full answer

Answered By

Rishabh Ojha
During my undergraduate i used to participate as TA (Teaching Assistant) in several electronics and computers subject. I'm passionate about learning Computer Science as my bachelors are in Electronics but i learnt most of the Computer Science subjects on my own which Machine Learning also. At Present, i'm a working professional pursuing my career as a Machine Learning Engineer and i want to help others learn during my free hours, that's all the motivation behind giving tuition. To be frank i have no prior experience of tutoring but i have solved problems on opensource platforms like StackOverflow and github. ~Thanks
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Operation Lucky Bag" was a sting operation used by New York City Police and Transit Police to reduce crime in the subway system. Would you consider the tactics of this operation entrapment or just...
-
Consider a salt of a weak base and a weak acid such as ammonium cyanide. Both the NH 4 + and CN ions interact with water in aqueous solution, but the net reaction can be considered as a proton...
-
The equilibrium constant for the reaction A B is Kc = 10 at a certain temperature. (1) Starting with only reactant A, which of the diagrams shown here best represents the system at equilibrium? (2)...
-
Return to Better Mousetraps in Problem 18. Suppose the firm can cut its requirements for working capital in half by using better inventory control systems. By how much will this increase project NPV?...
-
Explain how income can be manipulated when the specific identification inventory costing method is used.
-
The following is a list of manufacturing costs incurred by Denney Manufacturing Co. during July: Direct materials used....................................................... $21,000 Indirect...
-
Fantastic Sams and Defendants PSTEVO, LLC and Jeremy Baker entered into a franchise agreement pursuant to which Fantastic Sams granted PSTEVO a franchise to operate a Fantastic Sams Salon. According...
-
The following account balances relate to the stockholders' equity accounts of Hanshew Corp. at year-end. A small stock dividend was declared and issued in 2011. The market value of the shares was...
-
A government institutes a rule that schools are allowed to sell whatever foods it chooses, provided that those foods are not high in sugar. Such a ruling is aligned with which economic system?
-
Solid ammonium chloride, NH 4 Cl, reacts with solid sodium hydroxide to produce ammonia gas, water, and sodium chloride, NaCl. (a) Write a balanced equation for this reaction. (b) According to the...
-
When ammonia gas is dissolved in water, is the water behaving as an acid, as a base, or neither? Explain.
-
Discuss the various data collection methods described in this section.
-
follows; Your colleague advises use of an alternative regression model specification as Incons = Bo+B hhsize +2 income +3 bulb+u (2) where Incons = log(cons) i. ii. Estimate the above regression...
-
Elizabeth just graduated from Texas A&M. She would like to purchase a house in five (5) years. She plans to invest $500 each month for five (5) years into a mutual fund that offers a nominal twelve...
-
Green marketing refers to the development and marketing of products designed to minimize negative effects on the physical environment or to improve the environment. This exercise is designed to help...
-
the creation of transactions deposits by bank lending is known as? justify with some examples
-
Let's say you sell a women's product that is used by both my 90-year-old Mother and my 21-year-old niece. Same promotional program? Different? Why or why not? What promotional tools would you use?
-
Aside from its value in planning, why is it essential to do a budget forecast of sales, costs, and profit?
-
During 2012, Cheng Book Store paid $483,000 for land and built a store in Georgetown. Prior to construction, the city of Georgetown charged Cheng $1,300 for a building permit, which Cheng paid. Cheng...
-
Are the results of the Saybolt viscometer tests considered to be direct measurements of viscosity?
-
Does the Saybolt viscometer produce data related to a fluids dynamic viscosity or kinematic viscosity?
-
Which type of viscometer is prescribed by SAE for measurements of viscosity of oils at 100C?
-
Outline the three major strategies pursued by Canadian businesses. What implications do they have for the HR functions within the firms? Illustrate your answer with suitable examples. What four...
-
Draw a conceptual framework from Input to process and Output
-
In the principles of developing others, what should be considered conditions conducive to growth?

Study smarter with the SolutionInn App


