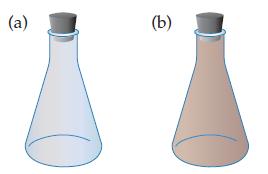
The reaction is run at one temperature in flask (a) and at a different temperature in flask
Question:
The reaction
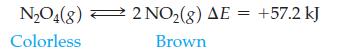
is run at one temperature in flask (a) and at a different temperature in flask (b). Which flask is at the lower temperature, and which is at the higher temperature? Explain your answer.
Transcribed Image Text:
N₂O4(8) 2 NO₂(g) AE = +57.2 kJ Colorless Brown
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Determining Temperature in Flasks The given information Reaction NO4g 2 NOg H 572 kJ endothermic Fla...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
GoPro has added the capability for recorded material to automatically sync between the cloud and the camcorder Which core marketing mix linkage does this represent Which supporting linkages could...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
A horizontal jet of water (at 10C) that is 6 cm in diameter and has a velocity of 20 m/s is deflected by the vane as shown. If the vane is moving at a rate of 7 m/s in the x-direction, what...
-
Making a Decision as an Auditor: Effects of Errors on Income, Assets, and Liabilities Megan Company (not a corporation) was careless about its financial records during its first year of operations,...
-
For each of the following multistep reactions, read the curved arrows and identify the sequence of arrowpushing patterns: a. b. c. d. e. :OH 0=s=0. :H 0=s=0: o=s=0:
-
Computing Unit Prices. Calculate the unit price of each of the following items.
-
The owner of an automobile repair shop studied the waiting times for customers who arrive at the shop for an oil change. The following data with waiting times in minutes were collected over a 1-month...
-
ter 1- Nature of Auditing (a) The primary responsibility for the prevention and detection of fraud rests with both those charged with governance of the entity and management. Explain
-
An acid is considered to be weak if: (a) It cannot neutralize a base. (b) It does not burn the skin. (c) Its equilibrium for dissociation lies far to the left. (d) It cannot produce hydronium (H 3 O...
-
Write a balanced dissociation equation for carbonic acid in water that shows the maximum number of H 3 O + ions the acid can yield.
-
When water is poured on the top of a pile of rocks, it always trickles down to the rocks on the bottom. Similarly, when rich people make lots of money, we can expect this money to trickle down to the...
-
2.11.2Project:Performance Task: The Parallax Problem Project Geometry Sem 1 (S3537251) Julio Duenas Points possible:120 Date: ____________ The Scenario:You're looking for a sponsor to pay for you to...
-
If the most common treatment of assigning overapplied overhead was used, the final balance in Cost of Goods Sold would have been * (1 Point) At the end of the last fiscal year, BREAD Company had the...
-
Angelina received new word processing software for her birthday. She also received a cheque with which she intends to purchase a new computer. Angelina's UNILUS Professor assigned a paper due in two...
-
At date t, the portfolio P to be hedged is a portfolio of Treasury bonds with various possible maturities. Its characteristics are as follows: Value YTM MD Convexity $1,450 6% 4.25 55 We consider...
-
A playground merry-go-round with an axis at the center (radius R = 1.3 m and rotational inertia | = 1.2 x 103 kgm2) is initially rotating at angular velocity w = 0.21 rad/s clockwise). A girl of mass...
-
The physical distribution channel of a major food manufacturer consists of plant stocks from which regional warehouses are restocked. These regional warehouses in turn supply the field warehouses...
-
Clark, PA, has been engaged to perform the audit of Kent Ltd.s financial statements for the current year. Clark is about to commence auditing Kents employee pension expense. Her preliminary enquiries...
-
How many yellow lightwaves ( = 580 nm) will fit into a distance in space equal to the thickness of a piece of paper (0.003 in.)? How far will the same number of microwaves (v = 10 10 Hz, i.e., 10...
-
Establish that where A, α, b, and c are all constant, is a solution of the differential wave equation. This is a Gaussian or bell-shaped function. What is its speed and direction of...
-
Argon-ion lasers typically generate multi-watt beams in the green or blue regions of the visible spectrum. Determine the frequency of such a 514.5-nm beam.
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


