Question: Write the complete ionic equation and the net ionic equation for the reaction that occurs when the following solutions are mixed, and name the salt
Write the complete ionic equation and the net ionic equation for the reaction that occurs when the following solutions are mixed, and name the salt formed in each case:
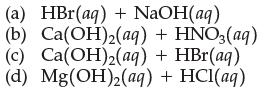
(a) HBr(aq) + NaOH(aq) (b) Ca(OH)2(aq) + HNO3(aq) (c) Ca(OH)(aq) + HBr(aq) (d) Mg(OH)2(aq) + HCl(aq)
Step by Step Solution
3.52 Rating (155 Votes )
There are 3 Steps involved in it
a HBraq NaOHaq Complete ionic equation Haq Braq Naaq OHaq Naaq Braq HOl Net ionic eq... View full answer

Get step-by-step solutions from verified subject matter experts


