Question: Write the equilibrium constant expression for (a) 2 FeCl3(s) + 3 HO(g) (b) FeO3(s) + 3 CO(g) (c) PbSO(s) Pb+ (aq) + SO3(aq) FeO3(s) +
Write the equilibrium constant expression for
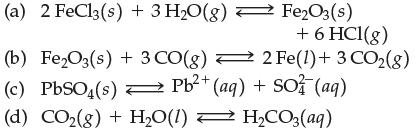
(a) 2 FeCl3(s) + 3 HO(g) (b) FeO3(s) + 3 CO(g) (c) PbSO(s) Pb+ (aq) + SO3(aq) FeO3(s) + 6 HCI(g) 2 Fe(1) + 3 CO(g) (d) CO(g) + HO(1) HCO3(aq) =
Step by Step Solution
3.41 Rating (151 Votes )
There are 3 Steps involved in it
a For the reaction 2FeCl3 s 3H2Og Fe2O3s 6HClg the equilibrium constant expression K is ... View full answer

Get step-by-step solutions from verified subject matter experts


