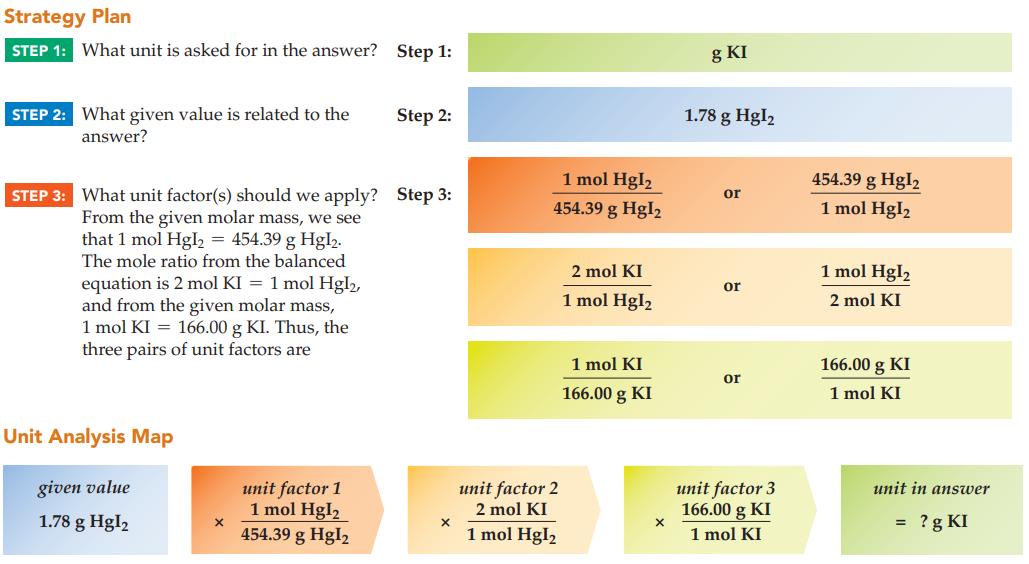
Calculate the mass of potassium iodide (166.00 g/mol) required to yield 1.78 g of mercury(II) iodide precipitate
Question:
Calculate the mass of potassium iodide (166.00 g/mol) required to yield 1.78 g of mercury(II) iodide precipitate (454.39 g/mol):
![]()
Transcribed Image Text:
2 KI(s) + Hg(NO3)2(ag) Hgl₂(s) + 2 KNO3(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
We select from each of the three pai...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Which is true regarding the bottom-up approach to budgeting expected project costs? A. The bottom-up approach allocates the overarching budget across the work packages. B. The bottom-up approach...
-
Calculate the mass of the precipitate formed when 2.27 L of 0.0820 M Ba(OH)2 are mixed with 3.06 L of 0.0664 M Na2SO4.
-
Mole Lab will rate answer Mole Laboratory Materials & Apparatus: Scale Graduated cylinder(s) Paper or plastic cups (8 oz or 16 oz) New container of small candy (jelly beans, Skittles, M&Ms,or...
-
Ebrahim Patel is a wholesaler who uses the periodic inventory system to account for inventory. Transactions for February: 1 Bought inventory from Rich Traders for R5 000 on credit. 2 Sold inventory...
-
Monsanto Company, a large chemical and fibers company, invested $37 million in state-of-the-art systems to improve process control, laboratory automation, and local area network (LAN) communications....
-
Hohenberger Farms purchased real estate for $1,280,000, which included $5,000 in legal fees. It paid $255,000 cash and incurred a mortgage payable for the balance. The real estate included land that...
-
prototype is an early sample, model, or release of a product that is built to test a concept or a process. The idea is to show the sample or model to potential users and then use their feedback to...
-
Paul Nasipak owns a business called Diamond Distributors. The following transactions took place during January of the current year. Journalize the transactions in a general journal using the periodic...
-
12. "New competition is not between what companies produce in their factories, but between what they add to their factory output in the form of packaging, services, advertising, customer advice,...
-
In general, how many unit factors are required to solve a massmass stoichiometry problem?
-
What mass of oxygen gas is produced from decomposing 5.00 g of water (18.02 g/mol)? 2 H 2 O(l) 2 H 2 (g) + O 2 (g) (a) 2.22 g (b) 2.50 g (c) 4.44 g (d) 5.00 g (e) 8.88 g.
-
Calculate the change in free energy for the transit of two electrons from the quinone pool to cytochrome c2 in bacterial photosynthetic electron transport. Assume that the reduction potential of...
-
Briefly describe the importance of trust,collegiality,fairness and accountability in research collaborations. Explanation and conclusion please
-
Someone once said, "failure to plan is a plan to fail". How might this apply to running a business? What is a feasibility study relative to a business plan? Describe the meaning of a "breakeven...
-
1. How would you describe the case study organizations strategy in international perspective? 2. What are the organizations reasons for adopting such a strategy? 3. Do you think it is a feasible...
-
Alfred wants to describe the overall response to each of the 9 statements. Which statistic should he use? Alfred wants to compare the factor score distributions for the controllers and users...
-
How would you describe the organizational culture of Alibaba? Motivate your answer by referring to organizational culture theory and in particular, the seven dimensions of organizational culture.
-
Zipcar (www.zipcar.com) is a car-sharing club founded in Cambridge, MA, in 1999. The club members pay an annual fee and then have the opportunity to rent a small car (usually a subcompact; the models...
-
What are the principal differences among asset liquidity management, liability management, and balanced liquidity management?
-
Companies that make no variable-cost/fixed-cost distinctions must use absorption costing, and those that do make variable-cost/fixed-cost distinctions must use variable costing. Do you agree? Explain.
-
The main trouble with variable costing is that it ignores the increasing importance of fixed costs in manufacturing companies? Do you agree? Why?
-
Give an example of how, under absorption costing, operating income could fall even though the unit sales level rises.
-
Hot Property Got to Be the Shoes - NIKE He rises into the air with his legs flared and a basketball held high. It's hard to forget the slam- dunk image of Michael Jordan - especially when it's...
-
Is the tax treatment in the scenario accurate for tax year 2020? The taxpayer was required to repay unemployment compensation that was received in a prior year. This may be a misce Yes No Click 'Yes'...
-
Can you answer question one detailed and witihin this case thank you In 1989 Hoppe married a Danish lawyer named Catrine, whose parents owned a small restaurant in Copenhagen. When Catrine's...

Study smarter with the SolutionInn App


