Question: Explain the decrease in solubility for the following acids in water. Acid CH3COOH acetic acid, pentanoic acid, C4HCOOH hexanoic acid, C5HCOOH decanoic acid, C9H19COOH Solubility
Explain the decrease in solubility for the following acids in water.
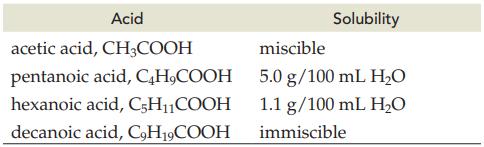
Acid CH3COOH acetic acid, pentanoic acid, C4HCOOH hexanoic acid, C5HCOOH decanoic acid, C9H19COOH Solubility miscible 5.0 g/100 mL HO 1.1 g/100 mL HO immiscible
Step by Step Solution
3.45 Rating (148 Votes )
There are 3 Steps involved in it
The decrease in solubility observed for the given acids in water can be explained by several factors including molecular structure polarity and interm... View full answer

Get step-by-step solutions from verified subject matter experts


