Hydrogen peroxide, H 2 O 2 , is prepared industrially from the reaction of oxygen and isopropyl
Question:
Hydrogen peroxide, H2O2, is prepared industrially from the reaction of oxygen and isopropyl alcohol, C3H7OH. Hydrogen peroxide reacts explosively with hydrazine, N2H4, and is used as a rocket fuel. The reactions for manufacturing hydrazine and the rocket explosion are as follows.
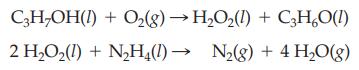
Starting with 50.0 mL of isopropyl alcohol (d = 0.786 g/mL) and excess other reactants, calculate:
(a) The mass of nitrogen gas produced
(b) The volume of nitrogen gas produced at STP
(c) The mass of water produced.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:





