If oxygen is collected over water at 20 C and 766 mm Hg, what is the partial
Question:
If oxygen is collected over water at 20 °C and 766 mm Hg, what is the partial pressure of the oxygen? Refer to Table 10.2 for the vapor pressure of water.
Table 10.2
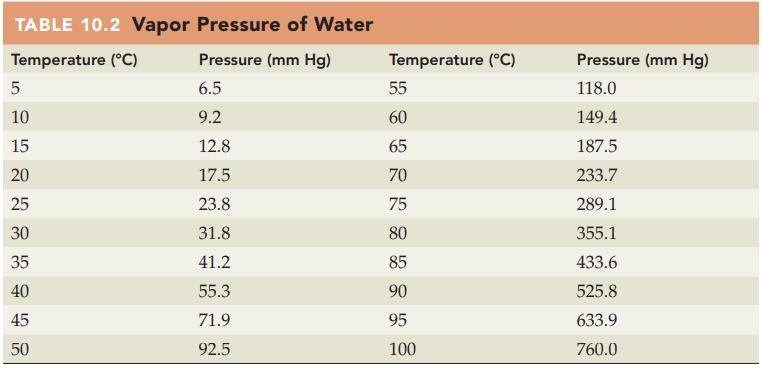
Transcribed Image Text:
TABLE 10.2 Vapor Pressure of Water Temperature (°C) Pressure (mm Hg) 6.5 9.2 12.8 17.5 23.8 31.8 41.2 55.3 71.9 92.5 сл 10 15 20 25 30 35 40 45 50 Temperature (°C) DRK 85 85 55 60 65 70 75 80 90 95 100 Pressure (mm Hg) 118.0 149.4 187.5 233.7 289.1 355.1 433.6 525.8 633.9 760.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The partial pressure of oxygen in the gas mixture collected over water at 20C and 766 mm Hg is 7485 ...View the full answer

Answered By

Joseph Ogoma
I have been working as a tutor for the last five years. I always help students to learn and understand concepts that appears challenging to them. I am always available 24/7 and I am a flexible person with the ability to handle a wide range of subjects.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
If ozone is collected over water at 30 C and 775 mm Hg, what is the partial pressure of the ozone? Refer to Table 10.2 for the vapor pressure of water. Table 10.2 TABLE 10.2 Vapor Pressure of Water...
-
If 75.0 mL of oxygen gas is collected over water at 20 C and 762 mm Hg, what is the pressure at 72.5 mL and 10 C? The vapor pressure of water at 10 C is 9.2 mm Hg.
-
Write from a perspective of a student learning econmoics, based on the songs lyrics of money- the beatles, answer the questions each in 4 sentences each What is an incentive? How does it relate to...
-
Calculate the standard entropy change for the following reactions at 25C. Comment on the sign of r S. (a) 2 Al(s) + 3 Cl 2 (g) 2 AlCl 3 (s) (b) 2 CH 3 OH() + 3 O 2 (g) 2 CO 2 (g) + 4 H 2 O(g)
-
Under Par, Inc., is an Internet retailer of golf equipment. Customers order golf equipment from the company, using an online catalog. The company processes these orders and delivers the requested...
-
Would it be more accurate to describe supply-siders as supply-and-demanders, who object to an overemphasis on aggregate demand to the neglect of effects on incentives to produce?
-
You throw a \(100-\mathrm{g}\) ball upward with a speed of \(19.8 \mathrm{~m} / \mathrm{s}\). How much work does the force of gravity do on the ball during its trip to its maximum height?
-
Consider the following condensed financial statements of Money Freedom, Inc. The companys target rate of return is 10% and its WACC is 7%: Requirements 1. Calculate the companys profit margin....
-
11. If a,b,x,ye R,@ #1, is a cube root of unity and (a+b)=x+yo, then (b+aw)' equals: (a) y+x@ 6 (c) -y-xw 12. The value of S = sin- -icos- (c) y+yw (d) -x-ya 2k is: 7 (c)-i (d) i , then value of...
-
Describe the meaning of the expression collecting a gas over water.
-
A steel cylinder with nitrogen, hydrogen, and ammonia gases is at 500 C and 5.00 atm. If the partial pressure of nitrogen is 1850 mm Hg and hydrogen is 1150 mm Hg, what is the partial pressure of...
-
Union Company is examining its operating cash management. One of the options the firm is considering is a zero-balance account (ZBA). The firms bank is offering a ZBA with monthly charges of $1,500,...
-
Question 1 Describe the research process to develop a business plan. Give some examples of legal, organisational and sustainability issues,and their potential business impacts you might need to...
-
QUESTION 47 Describe value analysis. What are the desirable outcomes of value analysis to the firm? To customers? QUESTION 48 What are the six types of focus? QUESTION 49 What are the five key...
-
PART 1 Describe at least 3 advertisements that have been persuasive in the way they communicate to their audience (the ads can be television, radio or via the internet). Provide the name of the ad...
-
Question 1(25p) Answer the questions based on the figure. (For this question, write your answer on a space left for you) A 1 2 B 7 8 4 3 C 2 4 1 D F E a) Traverse the graph using depth first search...
-
Company: Apple Describe the Team Leadership Model. Illustrate culture and leadership. Appraise the effects of gender, leadership style, and leadership effectiveness. Formulate the implications of...
-
Why might the term average cost be misleading?
-
The Home Depot is the leading retailer in the home improvement industry and one of the 10largest retailers in the United States. The company included the following on its January 29, 2012, balance...
-
What is a joint cost? What is a separable cost?
-
Distinguish between a joint product and a byproduct?
-
Why might the number of products in a joint-cost situation differ from the number of outputs? Give an example.
-
Given the variety of pediatric dentistry services offered, is there one service that has high (consistent) demand---always being produced every day from open to close---that when paired with a more...
-
3. If the question is whether a shift in demand will have a greater effect on equilibrium price or quantity, the answer lies not with the elasticity of demand, but with the elasticity of supply. Why...
-
Rational choice theory implicitly or explicitly assumes a number of things about consumer choice that are often not true, such as consumers search for an optimal solution to a problem and choose on...

Study smarter with the SolutionInn App


