Nitric acid is prepared industrially by the conversion of ammonia. There are three steps in the conversion,
Question:
Nitric acid is prepared industrially by the conversion of ammonia. There are three steps in the conversion, which is referred to as the Ostwald Process.
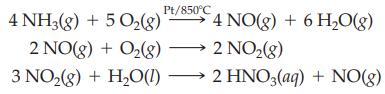
What is the mass of HNO3 (63.02 g/mol) produced, assuming 10.0 L of NH3 at STP is completely converted to nitric acid?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:





