Question: Various uses for nitric acid are given in Problem 6.43, along with information about how this important chemical is synthesized industrially. The key reactions are
Various uses for nitric acid are given in Problem 6.43, along with information about how this important chemical is synthesized industrially. The key reactions are oxidations of ammonia to nitric oxide and of nitric oxide to nitrogen dioxide, followed by dissolution of NO2 in water:
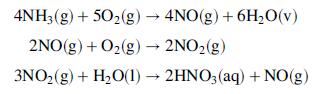
Nitric oxide generated on dissolution of NO2 in water is oxidized to produce additional NO2, which is then combined with water to form more HNO3. In this problem we neglect side reactions that would lower the product yield.
Ammonia vapor at 275°C and 8 atm is mixed with air, also at 275°C and 8 atm, and the combined stream is fed to a converter. Fresh air entering the system at 30°C and 1 atm with a relative humidity of 50% is compressed to 100°C and 8 atm, and the compressed air then exchanges heat with the product gas leaving the converter. The quantity of oxygen in the feed to the converter is 20% in excess of the amount theoretically required to convert all of the ammonia to HNO3. The entire process after the compressor may be taken to operate at a constant pressure of 8 atm.
In the converter, the ammonia is completely oxidized, with a negligible amount of NO2 formed.
The product gas leaves the converter at 850°C, and, as described in the preceding paragraph, exchanges heat with the air entering the system. The product gas then is fed to a waste-heat boiler that produces superheated steam at 200°C and 10 bar from liquid water at 35°C. The product gas leaving the wasteheat boiler is cooled further to 35°C and fed to an absorption column in which the NO is completely oxidized to NO2, which in turn combines with water some of which is present in the product gas.
Water is fed to the absorber at 25°C, at a rate sufficient to form a 55 wt% aqueous nitric acid solution. The NO formed in the reaction of NO2 to produce HNO3 is oxidized, and the NO2 produced is hydrated to form still more HNO3. The off-gas from the process may be taken to contain only N2 and O2.
(a) Construct a flowchart showing all process streams, including input and output from the process and the following equipment: converter, air compressor, exchanger recovering heat from the converter product, waste-heat boiler producing superheated steam, exchanger cooling the product gas before it is fed to the absorber, and absorber.
(b) Taking a basis of 100 kmol of ammonia fed to the process, develop spreadsheets (preferably incorporating the use of APEx) to determine the following:
(i) Molar amounts (kmol) of oxygen, nitrogen, and water vapor in the air fed to the process, cubic meters of air fed to the process, and kmol of water fed to the absorber.
(ii) Molar amounts, molar composition, and volume of the off-gas leaving the absorber.
(iii) Mass (kg) of product nitric acid solution.
(iv) Molar amounts and composition of the gas leaving the converter.
(v) Heat removed from or added to (state which) the converter.
(vi) Temperature of the product gas after it has exchanged heat with the air, assuming no heat is transferred between the heat exchanger and the surroundings.
(vii) Production rate of superheated steam if the gas temperature leaving the boiler is 205°C.
Before performing this calculation, determine if condensation of water occurs when the gas is cooled to 205°C. Since the superheated steam temperature is 200°C, explain why the selected temperature of the product gas is reasonable.
(viii) Heat removed from the product gas before it is fed to the absorber Check the condition of the gas at 35°C) and mass of cooling water required to remove that heat if the water temperature can only be increased by 5°C. Assume no heat is transferred between the heat exchanger and the surroundings.
(ix) Heat removed from or added to the absorber. Assume the heat capacity of the nitric acid solution is approximately the same as that of liquid water and the outlet temperatures of the off gas and product streams are 25°C and 35°C, respectively.
(c) Scale up the results calculated in Part (b) to determine all stream flow rates and heat transfer rates for a production rate of 5:0 × 103 kg/h of the product solution.
Problem 6.43.
Nitric acid is a chemical intermediate primarily used in the synthesis of ammonium nitrate, which is used in the manufacture of fertilizers. The acid also is important in the production of other nitrates and in the separation of metals from ores.
Nitric acid may be produced by oxidizing ammonia to nitric oxide over a platinum–rhodium catalyst, then oxidizing the nitric oxide to nitrogen dioxide in a separate unit where it is absorbed in water to form an aqueous solution of nitric acid.
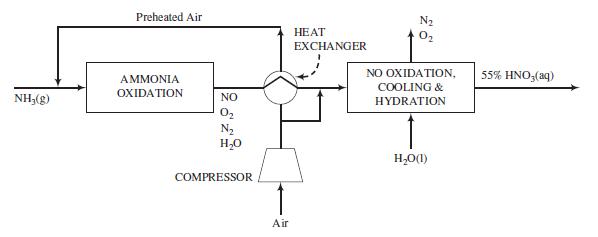
The reaction sequence is as follows:
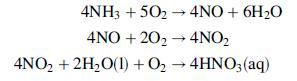
where, unless otherwise specified, the species are gases. A side reaction in which ammonia is oxidized to form nitrogen and water can lower product yield:
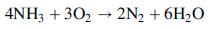
Ammonia vapor produced by vaporizing pure liquidammonia at 820 kPa absolute is mixed with air, and the combined streamenters the ammonia oxidation unit. Air at 30°C, 1 atmabsolute, and 50%relative humidity is compressed and fed to the process. A fraction of the air is sent to the cooling and hydration units, while the remainder is passed through a heat exchanger and mixed with the ammonia. The total oxygen fed to the process is the amount stoichiometrically required to convert all of the ammonia to HNO3,while the fraction sent to the ammonia oxidizer corresponds to the stoichiometric amount required to convert ammonia to NO.
The ammonia reacts completely in the oxidizer, with 97% forming NO and the rest forming N2. Only a negligible amount of NO2 is formed in the oxidizer. However, the gas leaving the oxidizer is subjected to a series of cooling and hydration steps in which the NO is completely oxidized to NO2, which in turn combines with water (some of which is present in the gas from the oxidizer and the rest is added) to form a 55 wt% aqueous solution of nitric acid. The product gas from the process may be taken to contain only N2 and O2.
Preheated Air N2 HEAT EXCHANGER 02 AMMONIA NO OXIDATION, 55% HNO,(aq) COOLING & OXIDATION NH3(g) NO HYDRATION N2 H,0 H,O(1) COMPRESSOR Air
Step by Step Solution
3.51 Rating (154 Votes )
There are 3 Steps involved in it
a In the compressor fresh air is fed at 30C and 1 atm with 50 relative humidity to be compressed to 100C and 8 atm Product gas from converter at 850C and 8 atm is used to heat the air from the compres... View full answer

Get step-by-step solutions from verified subject matter experts


