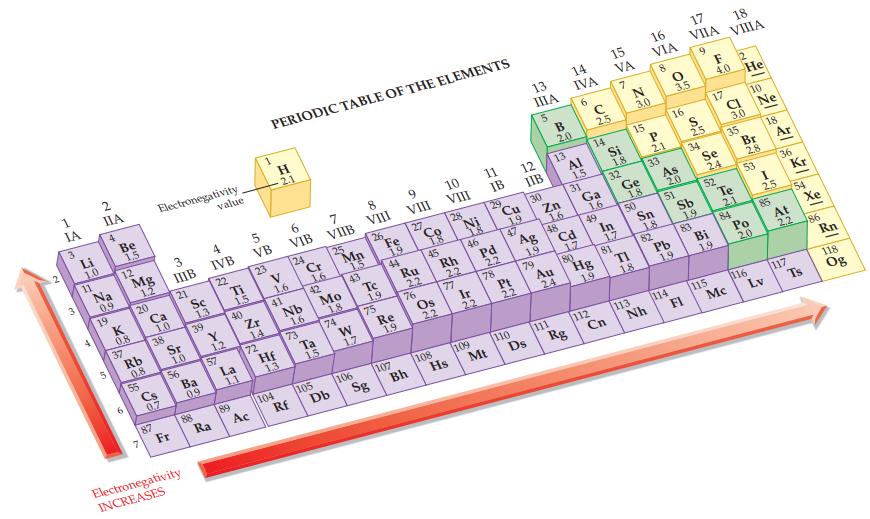
Refer to Figure 12.9 and label each atom in the following polar covalent bonds using delta notation
Question:
Refer to Figure 12.9 and label each atom in the following polar covalent bonds using delta notation (δ+ and δ–).
(a) C—H
(b) Se—O
(c) P—I
(d) H—Br.
Figure 12.9
Transcribed Image Text:
IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be 1,5 12 NE K 0.8 37 Mg 1.2 20 Rb 0.8 55 Ca 1.0 Electronegativity 38 Cs 0,7 87 3 IIIB 21 Sr 1.0 56 Fr Sc 1.3 39 Ba 0.9 Electronegativity INCREASES 88 value 4 IVB 22 Y 1.2 57 Ra Ti 15 89 40 La 1.1 5 VB 23 Zr 14 72 Ac PERIODIC TABLE OF THE ELEMENTS H 2.1 V 1.6 41 Hf 13 104 6 VIB 24 Nb 16 73 Rf Cr 1.6 42 Ta 1.5 7 VIIB 105 25 Mo 1.8 74 Db Mn 1.5 43 W 1,7 8 VIII 106 26 Te 19 75 Sg Fe 22 EN ON 19 44 Re 1.9 9 VIII 107 27 Ru 22 76 Bh Co 45 Os 22 10 VIII 28 108 Rh 22 77 Hs 18 46 Ir 22 11 IB 109 29 Pd 2.2 78 Mt Cu 19 47 Pt 22 110 13 ΠΙΑ 5 12 IIB 30 Ag 1.9 79 Ds B 2.0 Zn 13 16 48 Au 111 24 14 IVA 6 Al 1.5 31 Cd 17 Rg 80 с 2.5 14 Ga 1.6 49 112 Hg 19 15 VA 7 Si 1.8 Cn 32 In 1.7 81 Ge 21 1.8. 15 50 7 TI 18. 3.0 113 16 VIA Nh P 2.1 Sn 18 8 33 82 As 20 Pb 1.9 O 3.5 51 114 17 VIIA 9 16 S 25 34 Sb 1.9 FI 83 Se 24 Bi F He 4.0 17 52 115 19 18 VIIIA CI 3.0 35 Te 2.1 Mc 84 Br 10 2.8 53 Po 20 116 Ne 18 I 25 85 3 Lv Ar 36 At Kr 117 54 2.2 Xe Ts 86 Rn 118 Og
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Using delta notation the atoms in the following polar covalent bonds can be labeled as fol...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
278+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 12.9 and label each atom in the following polar covalent bonds using delta notation ( + and ). (a) HS (b) OS (c) NF (d) SCl. Figure 12.9 IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be 1,5...
-
From the trends in the periodic table, apply delta notation and label each atom in the following polar covalent bonds: (a) NO (b) BrF.
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Reference frame S is moving along the x axis at 0.6c relative to frame S. A particle that is originally at x = 10 m at t 1 = 0 is suddenly accelerated and then moves at a constant speed of c/3 in...
-
How do operational accountability and fiscal accountability differ? In what context are they used?
-
Greater than 0.18 In assume that a randomly selected subject is given a bone density test. Those test scores are normally distributed with a mean of 0 and a standard deviation of 1. In each case,...
-
Figure \(\mathrm{P} 27. 33\) shows the arrangement we looked at in Example 27. 2: a metal bar \(0.20 \mathrm{~m}\) long suspended from two springs, cach having a spring constant \(k=0.10 \mathrm{~N}...
-
Race One Motors is an Indonesian car manufacturer At its largest manufacturing facility, in Jakarta, the company produces subcomponents at a rate of 300 per day, and it uses these subcomponents at a...
-
31 (i) Explain why the refractive index of any material with respect to air is always greater 1. (ii) In the figure below a light ray travels from air into the semi-circular plastic block. Give a...
-
Refer to Figure 12.9 and indicate which of the following are nonpolar covalent bonds. (a) ClCl (b) ClN (c) NH (d) HP. Figure 12.9 IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5 Be 1,5 12 NE K 0.8 37 Mg 1.2 20...
-
Refer to the values in Figure 12.9 and calculate the electronegativity difference in each of the following bonds. (a) BrCl (b) BrF (c) ICl (d) IBr. Figure 12.9 IA 3 3 Li 1.0 11 4 IIA Bo Na 0.9 19 5...
-
A "green" product (e.g., a product built from recycled materials) is one that has minimal impact on the environment and human health. How do consumers determine if a product is "green"? The 2011...
-
Quiz 5 i 7 Mc Graw Hill 02-52-15 Type here to search Multiple Choice Operational control has a management-by-exception approach in contrast to management control, which is more consistent with: C A...
-
What is Evolution of Operating Systems? Explain
-
Assuming that we can change a person's attitude about a brand by 1) changing whether they think an important attribute of the brand is bad or good, 2) changing the weight given to an attribute or...
-
Calculate Company Mann's WACC Data Long term Debt 500 Cost of Debt Accounts Payable 100 Book Value of Equity 900 Cost of Equity WACC Risk Free Rate 3% Market Cap 700 Yield on Debt 8% Market Risk...
-
A company's bond has an annual coupon rate of 4 % . The bond pays coupons semiannually and has a par value of $ 1 , 0 0 0 . The bond matures in 6 years. What is the percentage ( % ) yield to maturity...
-
Return to the facts of problem 22. Assume that Mayfair Corporation exchanges its machine for another machine worth $18,000. How much boot must be paid to make the exchange, and who must pay the boot?...
-
The column shown in the figure is fixed at the base and free at the upper end. A compressive load P acts at the top of the column with an eccentricity e from the axis of the column. Beginning with...
-
Cash management what options are available to a firm if r believes it has too much cash? How about too little?
-
Motivations for holding cash in the chapter opening, we have discussed the enormous cash position of several companies. Why would firms such as these hold such large quantities of cash?
-
Cash management versus liquidity management what is the difference between cash management and liquidity management?
-
Internal Insights Inc., a developer of radiology equipment, has stock outstanding as follows: 70,000 shares of cumulative preferred 2% stock, $60 par, and 100,000 shares of $10 par common. During its...
-
Observe and analyze an organizational meeting ( board meeting, committee meeting ) and assess interdisciplinary patterns and chain of command. Highlight one interdepartmental discussion. What were...
-
Corey had been planning a family getaway for approximately eighteen months, to a large amusement park, approximately four hours away by car. About a year ago, he and his wife, Samantha, had to cancel...

Study smarter with the SolutionInn App


