Refer to Figure 17.4 and state whether each of the following ionic redox reactions is spontaneous or
Question:
Refer to Figure 17.4 and state whether each of the following ionic redox reactions is spontaneous or nonspontaneous.
(a) H+(aq) + I–(aq) → H2(g) + I2(s)
(b) Fe3+(aq) + I–(aq) → Fe2+(aq) + I2(s)
Figure 17.4
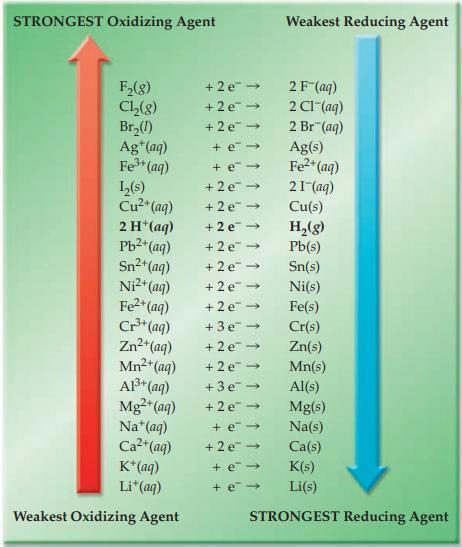
Transcribed Image Text:
STRONGEST Oxidizing Agent F₂(8) Ch₂(8) Br₂(1) Ag+ (aq) Fe³+ (aq) L₂(s) Cu²+ (aq) 2 H*(aq) Pb²+ (aq) Sn²+ (aq) Ni²+ (aq) Fe²+(aq) Cr³+ (aq) Zn²+ (aq) Mn²+ (aq) A1³+ (aq) Mg²+ (aq) Na* (aq) Ca²+ (aq) K+ (aq) Li+ (aq) Weakest Oxidizing Agent +2e™ → +2e → +2e → + e→ + e → +2e → +2e → +2e → +2e → +2e → +2e → +2e → +3e → +2e → +2e → +3e → +2e → + e- +2e → + e- + e- Weakest Reducing Agent 2 F (aq) 2 CI (aq) 2 Br(aq) Ag(s) Fe²+ (aq) 21- (aq) Cu(s) H₂(g) Pb(s) Sn(s) Ni(s) Fe(s) Cr(s) Zn(s) Mn(s) Al(s) Mg(s) Na(s) Ca(s) K(s) Li(s) STRONGEST Reducing Agent
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Determining whether a redox reaction is spontaneous or nonspontaneous involves assessing the standar...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 17.4 and state whether each of the following ionic redox reactions is spontaneous or nonspontaneous. (a) Mg(s) + Sn 2+ (aq) Mg 2+ (aq) + Sn(s) (b) H + (aq) + Ni(s) H 2 (g) + Ni 2+...
-
Refer to the National Highway Traffic Safety Administration (NHTSA) crash tests of new car models, presented in Exercise 2.190 (p. 107). Recall that the NHTSA has developed a "star" scoring system,...
-
State whether each of the following is true or false. a. The significance level of a test is the probability that the null hypothesis is false. b. A Type I error occurs when a true null hypothesis is...
-
Suppose you have a list of blood platelet counts from 500 patients in a hospital. Which of the following is most helpful in understanding the distribution of those values: frequency table, pie chart,...
-
Match the statement with the term most directly associated with it. Goodwill Intangible assets Research and development costs Amortization Franchise 1. _______ Rights, privileges, and competitive...
-
In Exercises, find the signs of the six trigonometric function values for the given angles. -57
-
A square loop of wire has a perimeter of \(4.00 \mathrm{~m}\) and is oriented such that two of its parallel sides form a \(25.0^{\circ}\) angle with the horizontal. A uniform horizontal magnetic...
-
Liberty Bell Fitness, Inc. operates a chain of fitness centers in Philadelphia. The firms controller is accumulating data to be used in preparing its annual profit plan for the coming year. The cost...
-
One key feature of Sage 50 Accounting software, it implements user access controls to restrict access to sensitive financial data. Maintain the security of financial information with password...
-
Sketch the following voltaic cell showing the two compartments, the anode and cathode, the wire connecting the two electrodes, and a salt bridge. Mn(s) + Cd(NO 3 ) 2 (aq) Cd(s) + Mn(NO 3 ) 2 (aq)
-
Refer to Figure 17.4 and state whether each of the following reactions is spontaneous or nonspontaneous. (a) Cr(s) + HCl(aq) CrCl 3 (aq) + H 2 (g) (b) FeCl 3 (aq) + NaI(aq) FeCl 2 (aq) + NaCl(aq) +...
-
Rollie exchanges a parking lot used in his business for a tract of land worth $20,000 and $4,000 in cash. He plans to subdivide and sell the land as residential lots. The adjusted basis of the...
-
If the $5 price described in the news clip were adopted, how would consumer surplus, producer surplus, and the deadweight loss change? The price of streaming services has been $10 a month or zero....
-
Calculate the slope of the relationship in Problem 4 between 3,846 and 4,071 theaters. Use the following news clip to work Problems. Kong Tops the Box Office Movie Kong: Skull Island Logan Get Out...
-
Let X U(a, b) a. Show that if \(x \leq a\) then \(P(X \leq x)=0\). b. Show that if \(x>b\) then \(P(X \leq x)=1\). c. Show that if \(a F(x) = 0 x-a b-a 1 x a a
-
Look at Figure 7.1 and follow the flow of transactions down the diagram. Then use the diagram to explain how costs are collected in the profit and loss account. Figure 7.1 Diagrammatic representation...
-
What have researchers found about the range of methods of overhead absorption used in practice?
-
I don't see any reason to do this net present value stuff. The payback method works well for me. It tells me how many years it takes to recoup the initial money I put in. As long as my payback period...
-
A bar of a steel alloy that exhibits the stress-strain behavior shown in Figure 6.22 is subjected to a tensile load; the specimen is 375 mm (14.8 in.) long and has a square cross section 5.5 mm (0.22...
-
A stockbroker advises a client to buy preferred stock. . . . With that type of stock, . . . [you] will never have to worry about losing the dividends. Is the broker right?
-
What are some of the factors that influence the market price of a corporations stock?
-
(a) What are the three conditions for the declaration and payment of a cash dividend? (b) The dates in connection with the declaration of a cash dividend are February 16, March 18, and April 17....
-
Amber has a Homeowners 3 policy. The dwelling is insured for $150,000 and the replacement cost of the home is $250,000. Indicate whether or not each of the following losses is covered. If possible,...
-
What is a social movement? How have the movements for racial and gender equality intersected with the labor movement? How do those movements intersect presently? What are some examples of the ways in...
-
What are the factors that may account for the decline in the overall unionization rates over the past 50 years? What are some of the distinctions between the public and private sector unionization...

Study smarter with the SolutionInn App


