Refer to Figure 17.4 and state whether each of the following ionic redox reactions is spontaneous or
Question:
Refer to Figure 17.4 and state whether each of the following ionic redox reactions is spontaneous or nonspontaneous.
(a) Mg(s) + Sn2+(aq) → Mg2+(aq) + Sn(s)
(b) H+(aq) + Ni(s) → H2(g) + Ni2+(aq)
Figure 17.4
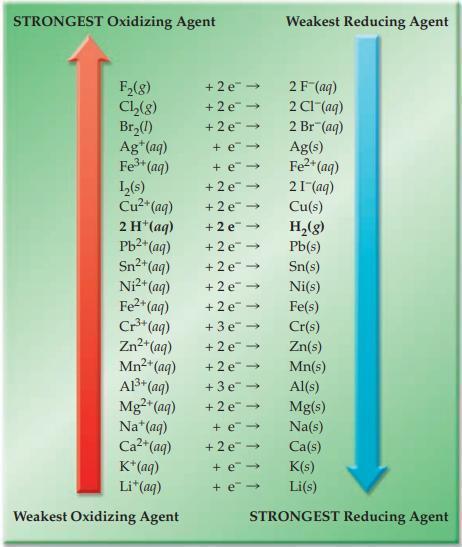
Transcribed Image Text:
STRONGEST Oxidizing Agent F₂(8) Ch₂(8) Br₂(1) Ag+ (aq) Fe³+ (aq) L₂(s) Cu²+ (aq) 2 H*(aq) Pb²+ (aq) Sn²+ (aq) Ni²+ (aq) Fe²+ (aq) Cr³+ (aq) Zn²+ (aq) Mn²+ (aq) A1³+ (aq) Mg²+ (aq) Na* (aq) Ca²+(aq) K+ (aq) Li+ (aq) Weakest Oxidizing Agent +2e™ → +2e → +2e → + e→ + e → +2e → +2e →> +2e → +2e → +2e → +2e → +2e →> +3e → +2e → +2e → +3e → +2e → + e- +2e → + e- + e- Weakest Reducing Agent 2 F (aq) 2 CI (aq) 2 Br(aq) Ag(s) Fe²+ (aq) 21- (aq) Cu(s) H₂(g) Pb(s) Sn(s) Ni(s) Fe(s) Cr(s) Zn(s) Mn(s) Al(s) Mg(s) Na(s) Ca(s) K(s) Li(s) STRONGEST Reducing Agent
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Spo...View the full answer

Answered By

Ajay Negi
Hi, I've completed my degree in engineering (Information Technology) from an NIT. Currently working as a software engineer. Wish to impart quality education to the future generation.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 17.4 and state whether each of the following ionic redox reactions is spontaneous or nonspontaneous. (a) H + (aq) + I (aq) H 2 (g) + I2(s) (b) Fe 3+ (aq) + I (aq) Fe 2+ (aq) + I...
-
Refer to the National Highway Traffic Safety Administration (NHTSA) crash tests of new car models, presented in Exercise 2.190 (p. 107). Recall that the NHTSA has developed a "star" scoring system,...
-
State whether each of the following statements is true or false, by reference to the relevant requirements of IFRS 8: 1. Company X must disclose EBITDA for each reportable segment. 2. Company X must...
-
Ask survey subjects to estimate the length of 1 minute without looking at a watch or clock. Each subject should say go at the beginning of the minute and then stop when he or she thinks that 1 minute...
-
Reinbold Manufacturing has an old factory machine that cost $50,000. The machine has accumulated depreciation of $28,000. Reinbold has decided to sell the machine. (a) What entry would Reinbold make...
-
In Exercises, find the signs of the six trigonometric function values for the given angles. -620
-
A circular loop of radius \(100 \mathrm{~mm}\) is placed in a magnetic field of magnitude \(0.030 \mathrm{~T}\). If the magnetic flux through the loop is \(3.00 \times 10^{-4} \mathrm{~T} \cdot...
-
The balance sheet caption for common stock is the following: Common stock without par value, 2,000,000 shares authorized, 400,000 shares issued, and 360,000 shares outstanding . . . . . . . . . . . ....
-
Question 1 Which class is needed to read data from the console? Input System Reader Scanner 1 pts
-
Sketch the following voltaic cell showing the two compartments, the anode and cathode, the wire connecting the two electrodes, and a salt bridge. Mn(s) + Cd(NO 3 ) 2 (aq) Cd(s) + Mn(NO 3 ) 2 (aq)
-
Refer to Figure 17.4 and state whether each of the following reactions is spontaneous or nonspontaneous. (a) Cr(s) + HCl(aq) CrCl 3 (aq) + H 2 (g) (b) FeCl 3 (aq) + NaI(aq) FeCl 2 (aq) + NaCl(aq) +...
-
Draw a plausible structure to represent: (a) [FeBr(ox) 2 ] 2 (b) [CoBr 4 (NH 3 ) 2 ] (c) [Cu(EDTA)] 2 (d) [CrBr 2 (H 2 O) 4 ] + (e) [PtBr 6 ] 2 .
-
What are the advantages of using block pulse operational transfer function (BPOTF) in control system analysis?
-
To implement an S/H device in practice what components do we need?
-
What are the advantages of using sample-and-hold operational transfer function (SHOTF) over the block pulse operational transfer function (BPOTF) in analyzing control systems involving...
-
Decompose the \((i+1)\) th block pulse function component using two delayed unit step functions, and graphically prove that the integration of these component functions finally result in the figure...
-
An account planner is often considered the consumer advocate. Pick a brand that you like and usea brand that would be your best friend. What three things does the consumer like most about the brand?...
-
What are some of the considerations that go into the choice of discount rate under the net present value method?
-
Recall that Chapter 8 described the binary search algorithm for finding a particular entry in an ordered list. The idea behind binary search is to begin looking in the exact center of the list. If...
-
An owner of 500 shares of Microshop Company common stock receives a stock dividend of 5 shares. (a) What is the effect of the stock dividend on the stockholders proportionate interest (equity) in the...
-
(a) Where should a declared but unpaid cash dividend be reported on the balance sheet? (b) Where should a declared but unissued stock dividend be reported on the balance sheet?
-
(a) In what respect does treasury stock differ from unissued stock? (b) How should treasury stock be presented on the balance sheet?
-
Employers who use independent contractors on a sufficiently exclusive basis may render them "dependent" contractors or even employees. How can employers minimize this risk? Explain briefly.
-
SSVS 1 applies when an AICPA member performs an engagement that estimates the value of a business, a business interest, security or intangible asset. Why it would make sense to split up SSVS 1 into...
-
fin609a he first step for this paper will be for you to select an appropriate company for your paper. Appropriate companies are U.S. based companies that have long-term debt on their balance sheets...

Study smarter with the SolutionInn App


