The chloride in an aqueous sample of BaCl 2 is precipitated with 50.0 mL of 0.100 M
Question:
The chloride in an aqueous sample of BaCl2 is precipitated with 50.0 mL of 0.100 M AgNO3. The excess silver nitrate is titrated with 17.0 mL of 0.125 M K2CrO4. Calculate the mass of the barium chloride in the sample.
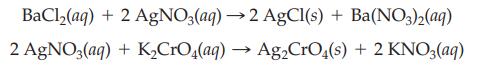
Transcribed Image Text:
BaCl₂(aq) 2 AgNO3(aq) + 2 AgNO3(aq) →2 AgCl(s) + Ba(NO3)2(aq) + K₂CrO4(aq) → Ag₂CrO4(s) + 2 KNO3(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
the balanced equation ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Silver nitrate reacts with strontium chloride in an aqueous precipitation reaction. What are the formulas of silver nitrate and strontium chloride? Write the molecular equation and net ionic equation...
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
Aoslia is a small country that takes the world price of corn as given. Its domestic supply and demand for corn are given by the following: a. Assume initially that Aoslia does not open to trade. What...
-
Reza Inc. operates a retail operation that purchases and sells snowmobiles, amongst other outdoor products. The company purchases all merchandise inventory on credit and uses a periodic inventory...
-
Convert from exponential form to logarithmic form. a. 10 2 = 100 b. 10x = 200 c. 10x = 0.05
-
Two parallel-plate capacitors are identical except that capacitor 1 has vacuum between the plates and capacitor 2 has a dielectric slab of dielectric constant \(\kappa\) filling the space between the...
-
Assigning activity-based costs in manufacturing, unused capacity, income Halifax Brass Company manufactures pumps and valves and uses a time-driven activity-based cost (TDABC) system. Last year,...
-
The price of a car you want is $39,000 today. Its price is expected to increase by $1000 each year. You now have $23,500 in an investment account, which is earning 11% per year. How many years will...
-
The president of a college reports to a board of trustees, and three vice-presidents report to the college president. The chief financial officer reports directly to the board of trustees. Draw a...
-
What is the molar chloride ion concentration resulting from mixing of 50.0 mL of 0.100 M sodium chloride and 25.0 mL of 0.100 M potassium chloride?
-
Consider the roller-coaster car in Problem 37. At what minimum value of the starting height \(b\) above the ground must the car begin its journey if it is to remain on the track at the top of the...
-
How may the effect of inflation be included in capital budgeting?
-
Explain how the liquid ratio is calculated.
-
Explain how the customers collection period is calculated.
-
What is zero-based budgeting?
-
Interviews will often be an intense experience in which you will be expected to be thorough and well prepared. Most of the time in an interview will be spent in answering questions that are asked of...
-
Suppose we are choosing between two drivers to allocate the costs in the perform setup cost pool: the number of setups or the number of setup hours. When will the choice not matter (i.e., will result...
-
All of the following assets can be depreciated, except: (a) A bulldozer (b) A copper mine (c) A surgical robot (d) A conveyor belt
-
Why should the timing of direct materials purchases be closely coordinated with the production budget?
-
a. Discuss the purpose of the cash budget. b. If the cash for the first quarter of the fiscal year indicates excess cash at the end of each of the first two months, how might the excess cash be used?
-
How does a schedule of collections from sales assist in preparing the cash budget?
-
Propylene glycol (C) is produced by the liquid-phase hydrolysis of propylene oxide (A). Sulfuric acid catalyzes the reaction. With excess water (B), the reaction is zero order with respect to water,...
-
1. Prepare any journal entry (if any) on the date of the restructured debt needed on Short's books (the Debtor). Otherwise, leave blank. Debit accounts Credit accounts Debits Credits Dr=Cr 2. Prepare...
-
Consider the following account balances (in thousands) for the Rouse Company: (Click the icon to view the account balances.) Requirements 1. Prepare a schedule for the cost of goods manufactured for...

Study smarter with the SolutionInn App


