The modern periodic law states that the properties of the elements repeat when the periodic table is
Question:
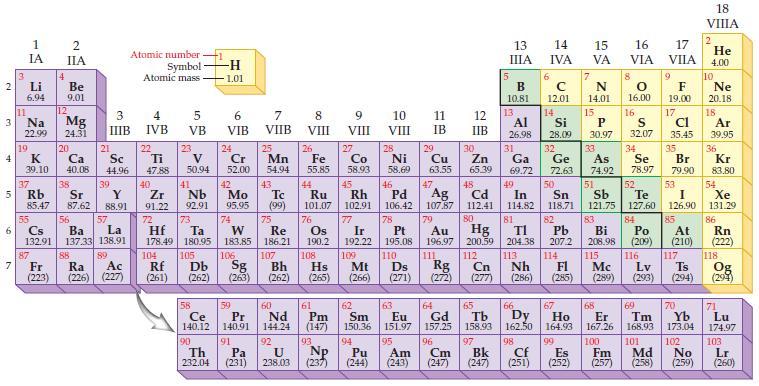
The modern periodic law states that the properties of the elements repeat when the periodic table is arranged according to which of the following?
(a) Increasing atomic number
(b) Increasing mass number
(c) Increasing atomic mass
(d) Increasing isotopic mass
(e) None of the above.
Periodic Table:
Transcribed Image Text:
2 3 4 10 6 3 7 11 1 IA Li 6.94 Na 22.99 19 37 5 Rb K 39.10 4 87 2 IIA Fr (223) Be 9.01 12 Mg 24.31 20 38 Ca Sc 40.08 44.96 21 Sr Y 85.47 87.62 88.91 55 56 La Cs Ba 132.91 137.33 138.91 88 3 4 IIIB IVB 39 57 89 Atomic number Symbol Atomic mass Ac Ra (226) (227) 22 Ti 47.88 5 VB 23 104 V 50.94 40 Nb Zr 91.22 92.91 41 105 -H 1.01 6 VIB 58 Ce 140.12 90 Th 232.04 24 Cr 52.00 42 Mo 95.95 74 72 73 W Re Hf Ta 178.49 180.95 183.85 186.21 106 Rf Db Sg (261) (262) (263) 59 Pr 140.91 91 7 VIIB Pa (231) 25 Mn 54.94 43 Tc (99) 75 107 60 Nd 144.24 8 VIII 92 26 27 Fe Co 55.85 58.93 Bh Hs (262) (265) 44 45 Ru Rh 101.07 102.91 76 Os 190.2 108 61 Pm (147) 9 VIII 93 U NP 238.03 (237) 77 Ir 192.22 109 Mt (266) 62 Sm 150.36 10 VIII 28 Ni 58.69 46 Pd 106.42 78 11 IB 94 95 Pu Am (244) (243) 13 IIIA 12 IIB 5 6 7 C 12.01 14 N 14.01 15 29 30 31 34 Si P 28.09 30.97 32 33 Cu Zn Ga Ge As Se 63.55 65.39 69.72 72.63 74.92 78.97 48 49 50 52 Ag Cd In Sn Sb Te 107.87 112.41 114.82 118.71 121.75 127.60 80 47 51 B 10.81 13 Al 26.98 14 15 IVA 16 VA VIA 79 81 82 Pt Au Hg TI Pb 195.08 196.97 200.59 204.38 207.2 110 111 112 113 114 Ds Rg Cn Nh Fl (271) (272) (277) (286) 83 Bi 208.98 115 16.00 16 52 S 32.07 84 Po (209) 17 VIIA 9 Md (258) F 19.00 17 Cl 35.45 35 Br 79.90 53 I 126.90 85 At (210) 116 Mc Lv (285) (289) (293) (294) Ts 117 66 70 63 64 65 67 68 69 Eu Gd Tb Dy Но Er Tm Yb 151.97 157.25 158.93 162.50 164.93 167.26 168.93 173.04 96 98 99 100 Cf Es Fm (251) (252) (257) 101 102 Cm (247) 97 Bk (247) 18. VIIIA 2 He 4.00 10 Ne 20.18 18 Ar 39.95 36 Kr 83.80 54 Xe 131.29 86 Rn (222) 118 Og (294) 71 Lu 174.97 103 No Lr (259) (260)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
The correct answer is a increasing atomic number The periodic table is arran...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Using the phrase structure rules given below, a) CP (C) TP b) TP {NP/CP} (T) VP c) VP (AdvP+) V (NP) ({NP/CP}) (AdvP+) (PP+) (AdvP+) d) NP (D) (AdjP+) N (PP+) (CP) e) PP P (NP) f) AdjP (AdvP)...
-
Parrot Company obtains some of the outstanding common stock of Sun Company on January 1, 2017. Parrot acquires Sun's 30% ownership interest for $250,000 in cash. Sun Company's balances are shown...
-
The sketch shows the right portion of a half-body modeling a small lake. The vertical line source of length 4 ft simulating water being drawn from the lake for a fountain at a volume rate of flow of...
-
What will be the value of x after executing the following java statement? Assume that value of x is 5 before the execution. x += x++ - 5;
-
Intermediate Sanctions. For each of the following independent situations, determine whether the organization is at risk for receiving intermediate sanctions from the Internal Revenue Service for...
-
(1) Define the term internal rate of return (IRR). What is each franchise's IRR? (2) How is the IRR on a project related to the YTM on a bond? (3) What is the logic behind the IRR method? According...
-
A gold cathode is illuminated with light of wavelength \(250 \mathrm{~nm}\). It is found that the current is zero when \(\Delta V=1.0 \mathrm{~V}\). Would the current change if a. The light intensity...
-
Consider the fuel cell stack of Problem 1.58. The t = 0.42 rum thick membranes have a nominal thermal conductivity of k = 0.79 W/m K that can be increased to keff,x = 15.1 W/m K by loading 10%, by...
-
Explain why a) an increase in free cash flow may also increase the agency cost of equity; and b) an increase in dividends or debt may be effective in making sure free cash flow are used in the best...
-
Why did Mendeleev not include argon in his periodic table of 1871? Periodic Table: 2 3 4 10 6 3 7 11 Li 6.94 1 IA Na 22.99 19 37 5 Rb K 39.10 al 55 Cs 132.91 87 4 Fr (223) 2 IIA Be 9.01 12 Mg 24.31...
-
Select the symbol of the element that fits each of the following descriptions: (a) The alkali metal in the fourth period (b) The halogen in the third period (c) The rare earth with the lowest atomic...
-
How do Venn diagrams show the effect of the three logical operators?
-
What is a trade secret? Provide an example of a trade secret, and describe how it helps a firm establish a competitive advantage in the marketplace.
-
Rick Sanford lives in a small community in northern Minnesota. He is planning to open the only fried chicken restaurant in his area and would like to trademark the words fried chicken. Because of his...
-
Shannon has developed a new type of space heater that is quieter and safer than previous generations of space heaters and is particularly geared to people who live in small spaces, such as apartments...
-
Cindy Coombs, a professional investor, was having lunch with a colleague recently and said, Do you remember Peter Kennedy, the entrepreneur we met the other day, who created an iPhone app that helps...
-
In start-up circles, its not uncommon to hear people say that they have a provisional patent or that theyre protected from someone stealing their invention because a provisional patent has been...
-
Use the following data for parts (a) through (g). a. Determine the equation of the simple regression line to predict y from x. b. Using the x values, solve for the predicted values of y and the...
-
As water moves through the hydrologic cycle, water quality changes are common because of natural phenomena or anthropogenic pollution. Using Figure 11.1, describe how water-quality changes occur...
-
Explain how the accounting for bad debts can be used for earnings management.
-
Because of calamitous earthquake losses, Bernstein Company, one of your clients oldest and largest customers, suddenly and unexpectedly became bankrupt. Approximately 30% of your clients total sales...
-
On January 1, 2010, Lombard Co. sells property for which it had paid $690,000 to Sergeant Company, receiving in return Sergeants zero-interest-bearing note for $1,000,000 payable in 5 years. What...
-
I am working on a Java project that uses comparable interfaces. This project is being done via Netbeans and is a project. Write a program that will create an array of Pet objects from a file of...
-
please explain answer Apply Johnson-trotter's method, find the next 5 permutations of the numbers below. 73 1 2 Paragraph BI UA/ Lato (Recom... V 19px... v 7 Ea Add a File Record Audin 8 5 C 32
-
la funcion definida en el ejercicio 4 y 5 deben ser usadas en este problema las puedes definir con el nombre que quieras y yo las sustituyo 6. (2 points) Create a function called best model that...

Study smarter with the SolutionInn App


