Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5
Question:
Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information.
(a) AgNO3(aq) + KI(aq) → AgI(s) + KNO3(aq)
(b) BaCl2(aq) + K2CrO4(aq) → BaCrO4(s) + KCl(aq)
Table 14.5
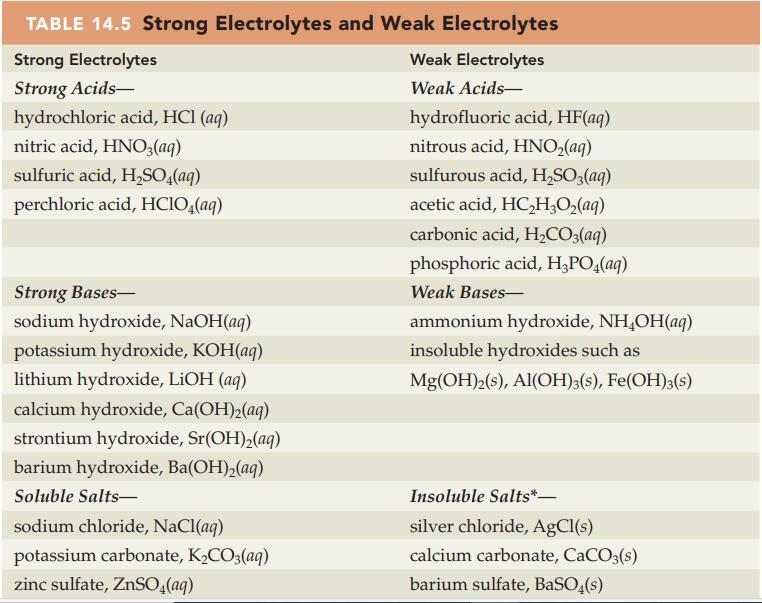
Transcribed Image Text:
TABLE 14.5 Strong Electrolytes and Weak Electrolytes Strong Electrolytes Strong Acids- hydrochloric acid, HCl(aq) nitric acid, HNO3(aq) sulfuric acid, H₂SO4(aq) perchloric acid, HClO4(aq) Strong Bases- sodium hydroxide, NaOH(aq) potassium hydroxide, KOH(aq) lithium hydroxide, LiOH (aq) calcium hydroxide, Ca(OH)₂(aq) strontium hydroxide, Sr(OH)₂(aq) barium hydroxide, Ba(OH)₂(aq) Soluble Salts- sodium chloride, NaCl(aq) potassium carbonate, K₂CO3(aq) zinc sulfate, ZnSO4(aq) Weak Electrolytes Weak Acids- hydrofluoric acid, HF(aq) nitrous acid, HNO₂(aq) sulfurous acid, H₂SO3(aq) acetic acid, HC₂H₂O₂(aq) carbonic acid, H₂CO3(aq) phosphoric acid, H₂PO4(aq) Weak Bases- ammonium hydroxide, NH₂OH(aq) insoluble hydroxides such as Mg(OH)2(s), Al(OH)3(s), Fe(OH)3(s) Insoluble Salts*- silver chloride, AgCl(s) calcium carbonate, CaCO3(s) barium sulfate, BaSO4(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Ag aq I a...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) Zn(NO 3 ) 2 (aq) + NaOH(aq) Zn(OH) 2 (s) + NaNO...
-
Write a balanced net ionic equation for each of the following reactions: (a) Dilute nitric acid reacts with zinc metal with formation of nitrous oxide. (b) Concentrated nitric acid reacts with sulfur...
-
Write a balanced net ionic equation for each of the following acidbase reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) HF(aq) + Li 2 CO 3 (aq) LiF(aq) + H 2 O(l) + CO...
-
Find the exact value of sin(x - y) if sin(x) = 3T 3T T
-
The current price of a stock is $33, and the annual risk-free rate is 6%. A call option with a strike price of $32 and with 1 year until expiration has a current value of $6.56. What is the value of...
-
In Exercises find the capitalized cost C of an asset (a) For n = 5 years (b) For n = 10 years (c) Forever. The capitalized cost is given by where C 0 is the original investment, t is the time in...
-
Sinclair Corp. manufactures radiation-shielding glass panels. Suppose Sinclair is considering spending the following amounts on a new TQM program: Sinclair expects the new program to save costs...
-
1. Andersons bank requires a compensating balance of $3 million. How much additional funds can be freed up for investment in fixed assets if the firm reduces its cash balance to the minimum required...
-
Other than the wind speed, what factor has the most impact on the amount of power generated by a wind turbine? Swept area of rotor Turbine style Terrain Blade material.
-
A drop of methyl red and a drop of phenolphthalein are added to a beaker of distilled water. What is the resulting color of the water?
-
What term describes ions in a total ionic equation that do not react?
-
Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C).
-
What is the difference between accepting and ignoring a risk? Why is risk ignorance not a suitable risk response?
-
The financial accounting department of CraftCo Production Inc. has provided you with the balance sheet and income statement figures that present the essential annual financial information. Determine...
-
Which cost components are included in total delivered cost (TDC)?
-
Leymann Ltd. is a multinational enterprise with over 1600 employees, which has its headquarter in Cologne, Germany. Founded in 1850, this company has been a pioneer in vacuum technologies. Today,...
-
Describe current shortcomings of digital twins in supply chain management accounting.
-
Using the standard costs in SE 3 and the following actual cost and usage data, compute the direct labor rate and direct labor efficiency variances: Direct labor hours used ...........4,950 hours...
-
Fred Farmer needs to prepare a balance sheet for his bank. He spent the day getting the following information. Fred needs your help to build a balance sheet and evaluate it. The information was...
-
Special Order Louisville Corporation produces baseball bats for kids that it sells for $32 each. At capacity, the company can produce 50,000 bats a year. The costs of producing and selling 50,000...
-
Contribution approach, relevant costs . Air Frisco has leased a single jet aircraft that it operates between San Francisco and the Fijian Islands. Only tourist-class seats are available on its...
-
Relevant costs, opportunity costs. Larry Miller, the general manager of Basil Software, must decide when to release the new version of Basils spreadsheet package, Easyspread 2.0. Development of...
-
The salmon in Steelfin Lake decrease their numbers by approximately 20% each year. To counteract this, the Steelfin Lake Conservation Society adds s salmon to the lake at the end of each year. Assume...
-
Assignment - Accounting Cycle Yellow Dog Company (YDC) is wholesale distributor in British Columbia. YDC has a December 31 year- end. The company is planning a major expansion next year that will...
-
How does Functionalism approach the analysis of social change and adaptation, particularly in understanding the processes by which societies evolve and adapt to internal and external pressures while...

Study smarter with the SolutionInn App


