Question: Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information. (a) Zn(NO
Write a balanced net ionic equation for each of the following solution reactions. Refer to Table 14.5 and Appendix D for electrolyte information.
(a) Zn(NO3)2(aq) + NaOH(aq) → Zn(OH)2(s) + NaNO3(aq)
(b) MgSO4(aq) + NH4OH(aq) → Mg(OH)2(s) + (NH4)2SO4(aq)
Table 14.5
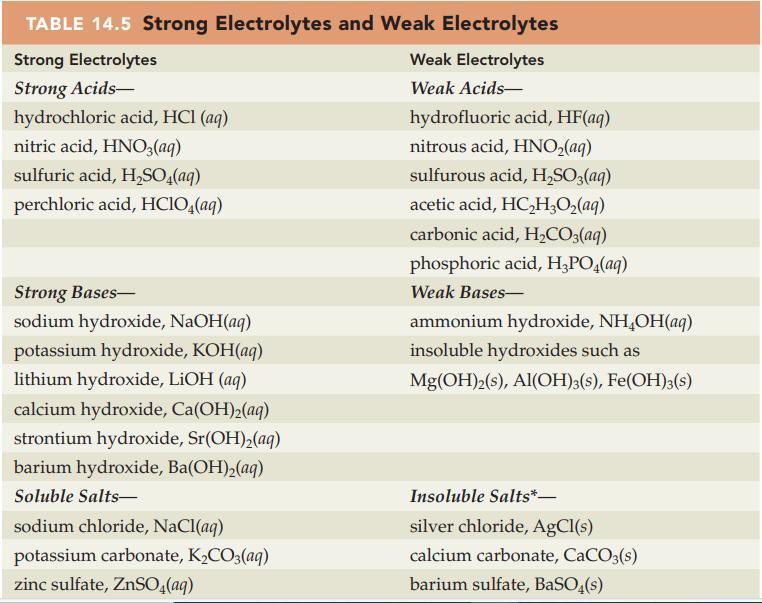
TABLE 14.5 Strong Electrolytes and Weak Electrolytes Strong Electrolytes Strong Acids- hydrochloric acid, HCl(aq) nitric acid, HNO3(aq) sulfuric acid, HSO4(aq) perchloric acid, HClO4(aq) Strong Bases- sodium hydroxide, NaOH(aq) potassium hydroxide, KOH(aq) lithium hydroxide, LiOH (aq) calcium hydroxide, Ca(OH)(aq) strontium hydroxide, Sr(OH)(aq) barium hydroxide, Ba(OH)(aq) Soluble Salts- sodium chloride, NaCl(aq) potassium carbonate, KCO3(aq) zinc sulfate, ZnSO4(aq) Weak Electrolytes Weak Acids- hydrofluoric acid, HF(aq) nitrous acid, HNO(aq) sulfurous acid, HSO3(aq) acetic acid, HCHO(aq) carbonic acid, HCO3(aq) phosphoric acid, HPO4(aq) Weak Bases- ammonium hydroxide, NHOH(aq) insoluble hydroxides such as Mg(OH)2(s), Al(OH)3(s), Fe(OH)3(s) Insoluble Salts*- silver chloride, AgCl(s) calcium carbonate, CaCO3(s) barium sulfate, BaSO4(s)
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
Completed and balanced ch... View full answer

Get step-by-step solutions from verified subject matter experts


