Question: Concern the crystal lattice for titanium, which has the hexagonal structure shown on the left in the accompanying figure. The vectors in R 3 form
Concern the crystal lattice for titanium, which has the hexagonal structure shown on the left in the accompanying figure. The vectors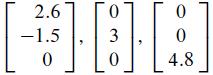
in R3 form a basis for the unit cell shown on the right. The numbers here are Angstrom units (1 A = 10-8 cm). In alloys of titanium, some additional atoms may be in the unit cell at the octahedral and tetrahedral sites (so named because of the geometric objects formed by atoms at these locations).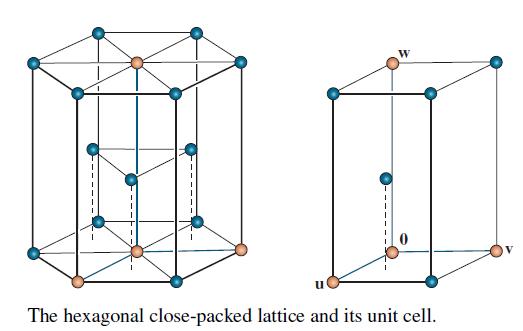
One of the tetrahedral sites is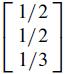
Determine the coordinates of this site relative to the standard basis of R3.
2.6 -1.5 0 0 3 0 0 0 4.8
Step by Step Solution
3.55 Rating (165 Votes )
There are 3 Steps involved in it
12 We are given that x3 12 where B 13 ... View full answer

Get step-by-step solutions from verified subject matter experts


