Concern the crystal lattice for titanium, which has the hexagonal structure shown on the left in the
Question:
Concern the crystal lattice for titanium, which has the hexagonal structure shown on the left in the accompanying figure. The vectors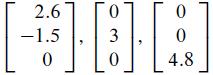
in R3 form a basis for the unit cell shown on the right. The numbers here are Angstrom units (1 A = 10-8 cm). In alloys of titanium, some additional atoms may be in the unit cell at the octahedral and tetrahedral sites (so named because of the geometric objects formed by atoms at these locations).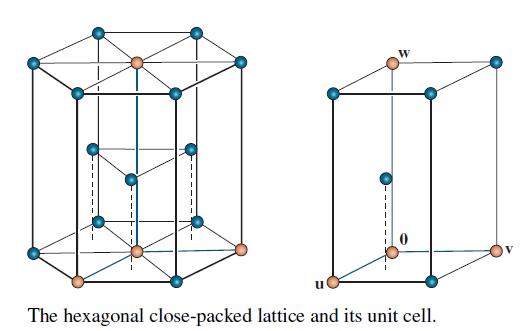
One of the octahedral sites is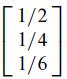
relative to the lattice basis. Determine the coordinates of this site relative to the standard basis of R3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Linear Algebra And Its Applications
ISBN: 9781292351216
6th Global Edition
Authors: David Lay, Steven Lay, Judi McDonald
Question Posted:





