An nth-order chemical reaction with one reactant obeys the differential equation dc -kc, dt ||
Question:
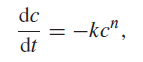
where c is the concentration of the reactant and k is a constant. Solve this differential equation by separation of variables. If the initial concentration is c0 moles per liter, find an expression for the time required for half of the reactant to react.
Transcribed Image Text:
dc -kc", dt ||
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (14 reviews)
For half of the original amo...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
A reactant in a first-order chemical reaction without back reaction has a concentration governed by the same formula as radioactive decay, where [A] 0 is the concentration at time t = 0, [A] t is the...
-
A reactant in a first-order chemical reaction without back reaction has a concentration governed by the same formula as radioactive decay, where [A] 0 is the concentration at time t = 0,[A] t is the...
-
In a second-order chemical reaction involving one reactant and having no back reaction, Solve this differential equation by separation of variables. Do a definite integration from t = 0 to t = t 1 ....
-
Nisha has completed her MBA and has joined a company which was going to raise fund from long term sources such as Debt and Equity. Nisha was asked by her manager to prepare a report on which could be...
-
Joan's Grocery Store made the following Form 941 payroll tax deposits during the look-back period of July 1, 201A, through June 30, 201B: Quarter Ended .................................. Amount Paid...
-
Review Conceptual Example 2 as an aid in solving this problem. An object is attached to the lower end of a 100-coil spring that is hanging from the ceiling. The spring stretches by 0.160 m. The...
-
How would you distinguish objects from classes? Provide an example. a. Comment on the statement: A class is an abstraction of objects. b. Comment on the statement: A class is a template for creating...
-
A partial adjusted trial balance for Orlando Company is given in E4-13. Instructions Prepare the closing entries at January 31, 2010.
-
(7) You have a DC motor that has a gain of 40 rad/s/V and a time constant of 0.25 s. Assume you apply a 2 V step input to the motor at time 0 s with an initial step value of 0 V. What would be the...
-
The 450-room Hotel Fernando is a four-star full-service hotel in San Petresco. It is highly regarded among both locals and tourists. Its service standards and ameni- ties complement its exquisite...
-
If z c (t) is a general solution to the complementary equation and zp(t) is a particular solution to the inhomogeneous equation, show that z c + z p is a solution to the inhomogeneous equation of Eq....
-
Locate the time at which z attains its maximum value and find the maximum value.
-
Match each of the key terms with the definition that best fits it. ____________ The amount of time that an activity can be delayed without delaying the project. Here are the key terms from the...
-
Islands tend to have fewer species than an equally sized area of the mainland. Is this consistent with the idea that species were spread around Earth purposefully? Is it consistent with evolution?
-
Why are some people skeptical that the supposed Martian fossils are of bacteria?
-
What is variation?
-
Duchennes muscular dystrophy is a condition that affects many more males than females. Knowing only this, how do you think the condition is inherited?
-
A population of beetles that includes both sandy and green individuals is introduced into a grassy environment. How do you expect the population to evolve due to natural selection? Now suppose that...
-
Instead of 40, if 60 of the 100 people had stated that the main reason they had visited the store was because the store is running a sale on coats that week a. The width of the 95% confidence...
-
Digital Fruit is financed solely by common stock and has outstanding 25 million shares with a market price of $10 a share. It now announces that it intends to issue $160 million of debt and to use...
-
If we focus on patients where there are different results for the supine and upright images [i.e., groups (iii) and (iv) above], what test can be performed to assess whether there is a significant...
-
Perform the test in Problem 10.17 and report a p-value (two-tailed). Interpret the results in words? Cardiology, Radiology The conventions of cardiac echocardiography are derived from comprehensive...
-
Perform the test in Problem 10.17 and report a p-value (two-tailed). Interpret the results in words? Cardiology, Radiology The conventions of cardiac echocardiography are derived from comprehensive...
-
The following information is available for ADT Company, which produces special-order security produc order costing system. Overhead is applied using a predetermined overhead rate of 55% of direct...
-
Stahelin Valves produces a single component, a valve. The valve sells for $43 per unit. Fixed costs are $1,605,000 annually. Production and sales of 407,000 units annually results in profit before...
-
Calaveras Tire exchanged equipment for two pickup trucks. The book value and fair value of the equipment given up were $29,000 (original cost of $78,500 less accumulated depreciation of $49,500) and...

Study smarter with the SolutionInn App


