Question: Radioactive nuclei decay according to the same differential equation which governs first-order chemical reactions, Eq. (12.71). In living matter, the isotope 14 C is continually
Radioactive nuclei decay according to the same differential equation which governs first-order chemical reactions, Eq. (12.71). In living matter, the isotope14C is continually replaced as it decays, but it decays without replacement beginning with the death of the organism. The half-life of the isotope (the time required for half of an initial sample to decay) is 5730 years. If a sample of charcoal from an archaeological specimen exhibits 0.97 disintegrations of14C per gram of carbon per minute and wood recently taken from a living tree exhibits 15.3 disintegrations of14C per gram of carbon per minute, estimate the age of the charcoal.
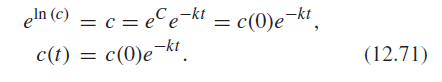
eln (c) = c = eCe-kt = c(0)e-kt, In (c) c = eCekt c(t) = c(0)ekt. c(0)ekt, (12.71)
Step by Step Solution
3.44 Rating (163 Votes )
There are 3 Steps involved in it
The rate of disintegrations is ... View full answer

Get step-by-step solutions from verified subject matter experts


