The van der Waals equation of state gives better accuracy than the ideal gas equation of state.
Question:
The van der Waals equation of state gives better accuracy than the ideal gas equation of state. It is
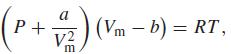
where a and b are parameters that have different values for different gases and where Vm = V/n, the molar volume. For carbon dioxide, a = 0.3640 Pa m6 mol–2, b = 4.267 × 10–5 m3 mol–1. Calculate the pressure of carbon dioxide in pascals, assuming that n = 0.13678 mol, V = 10.00l, and T = 298.15 K. Convert your answer to atmospheres and torr.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





