Question: (a) Consider H 2 O in contact with a heat and volume bath with temperature T and pressure P. For certain values of T and
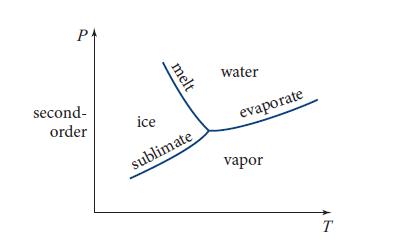
(a) Consider H2O in contact with a heat and volume bath with temperature T and pressure P. For certain values of T and P the H2O will be liquid water; for others, ice; for others, water vapor—and for certain values it may be a two- or three-phase mixture of water, ice, and/or vapor. Show, using the Gibbs potential and its Euler equation, that, if two phases a and b are present and in equilibrium with each other, then their chemical potentials must be equal: μa = μb. Explain why, for any phase a, μa is a unique function of T and P. Explain why the condition μa = μb for two phases to be present implies that the two-phase regions of the T -P plane are lines and the three-phase regions are points (see Fig. 5.6). The three-phase region is called the “triple point.” The volume V of the two- or three phase system will vary, depending on how much of each phase is present, since the density of each phase (at fixed T and P) is different.
b. Show that the slope of the ice-water interface curve in Fig. 5.6 (the “melting curve”) is given by the Clausius-Clapeyron equation
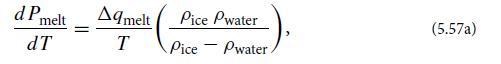
where ρ is density (mass per unit volume), and △qmelt is the latent heat per unit mass for melting (or freezing)—the amount of heat required to melt a unit mass of ice, or the amount released when a unit mass of water freezes. Notice that, because ice is less dense than water, the slope of the melting curve is negative.
(c) Suppose that a small amount of water is put into a closed container of much larger volume than the water. Initially there is vacuum above the water’s surface, but as time passes some of the liquid water evaporates to establish vapor-water equilibrium. The vapor pressure will vary with temperature in accord with the Clausius-Clapeyron equation.
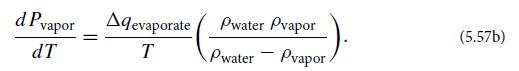
Now suppose that a foreign gas (not water vapor) is slowly injected into the container. Assume that this gas does not dissolve in the liquid water. Show that, as the pressure Pgas of the foreign gas gradually increases, it does not squeeze water vapor into the water, but rather it induces more water to vaporize:
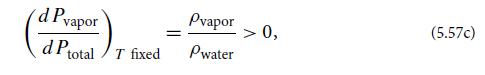
where Ptotal = Pvapor + Pgas
Figure 5.6
dPm melt dT = Aqmelt T Pice Pwater Pice Pwater. - (5.57a)
Step by Step Solution
3.27 Rating (147 Votes )
There are 3 Steps involved in it
a In a system where two phases a and b of a substance eg HO are in equilibrium with each other under a given temperature T and pressure P the Gibbs potential also known as Gibbs free energy G is minim... View full answer

Get step-by-step solutions from verified subject matter experts


