Question: Moseley pointed out that elements with atomic numbers 43, 61, and 75 should exist and (at that time) had not been found. (a) Using Figure
Moseley pointed out that elements with atomic numbers 43, 61, and 75 should exist and (at that time) had not been found.
(a) Using Figure 4-19, what frequencies would Moseley’s graphical data have predicted for the Ka x ray for each of these elements?
(b) Compute the wavelengths for these lines predicted by Equation 4-37.
Figure 4-19
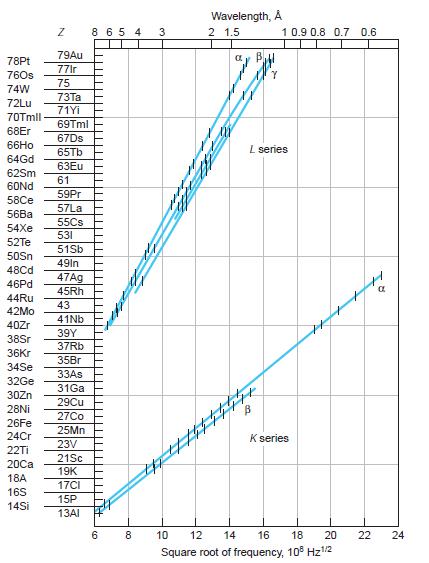
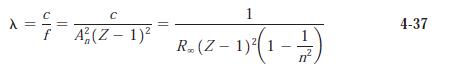
78Pt 760s 74W 72Lu 70Tmll 68Er 66Ho 64Gd 62Sm 60Nd 58Ce 56Ba 54Xe 52Te 50Sn 48Cd 46Pd 44Ru 42Mo 40Zr 38Sr 36Kr 34Se 32Ge 30Zn 28Ni 26Fe 24Cr 22Ti 20Ca 18A 16S 14Si N 79Au 77lr 75 73Ta 71Yi 69Tml 67Ds 65Tb 63Eu 61 59Pr 57La 55Cs 531 51Sb 49In 47Ag 45Rh 43 41Nb 39Y 37Rb 35Br 33As 31Ga 29Cu 27Co 25Mn 23V 21Sc 19K 17CI 15P 13AI 8 6 5 4 6 8 3 Wavelength, A 2 1.5 ay B 1 0.9 0.8 0.7 0.6 L series H B K series 10 12 14 16 18 20 Square root of frequency, 108 Hz/2 22 24
Step by Step Solution
3.44 Rating (170 Votes )
There are 3 Steps involved in it
a Note f 12 for Z 61 and 75 are off the graph 419 however ... View full answer

Get step-by-step solutions from verified subject matter experts


