Question: Use the data from Table 9-8 to find the force constant for (a) The H 35 Cl and (b) The K 79 Br molecules. Table
Use the data from Table 9-8 to find the force constant for
(a) The H35Cl and
(b) The K79Br molecules.
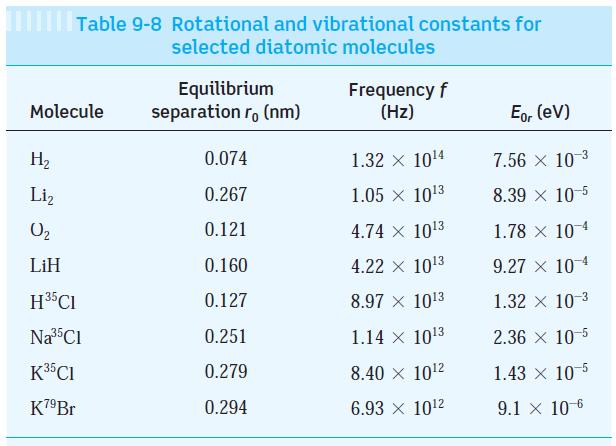
Table 9-8 Rotational and vibrational constants for selected diatomic molecules Molecule H Liz 0 LiH H5Cl Na 5C1 K35Cl K7Br Equilibrium separation r, (nm) 0.074 0.267 0.121 0.160 0.127 0.251 0.279 0.294 Frequency f (Hz) 1.32 104 1.05 103 4.74 103 4.22 x 103 8.97 1013 1.14 103 8.40 102 6.93 X 102 Eor (ev) 7.56 10-3 8.39 x 10-5 1.78 x 104 9.27 x 10-4 1.32 x 10-3 2.36 x 10-5 1.43 x 10-5 9.1 x 10-6
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
Equation 921 a For H 35 Cl 0980u ... View full answer

Get step-by-step solutions from verified subject matter experts


