Question: Using data from Table 9-8, (a) Compute the vibrational energy of the LiH molecule in its lowest vibrational state. (b) Compute the reduced mass of
Using data from Table 9-8,
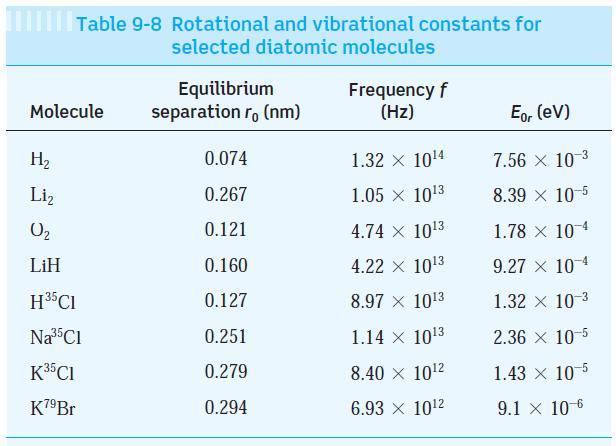
(a) Compute the vibrational energy of the LiH molecule in its lowest vibrational state.
(b) Compute the reduced mass of LiH.
(c) Determine the force constant for LiH.
(d) From those results, compute an estimate of the LiH bond length and compare your result with the value in the table.
Table 9-8 Rotational and vibrational constants for selected diatomic molecules Molecule H Liz 0 LiH H5Cl Na 5C1 K35 Cl K7Br Equilibrium separation (nm) 0.074 0.267 0.121 0.160 0.127 0.251 0.279 0.294 Frequency f (Hz) 1.32 104 1.05 103 4.74 103 4.22 x 103 8.97 1013 1.14 103 8.40 102 6.93 X 102 Eor (ev) 7.56 10-3 8.39 x 10-5 1.78 x 104 9.27 x 104 1.32 x 10-3 2.36 x 10-5 1.43 x 10-5 9.1 x 10-6
Step by Step Solution
3.52 Rating (155 Votes )
There are 3 Steps involved in it
For LiH f 422 x 10 Hz from Table 97 a ... View full answer

Get step-by-step solutions from verified subject matter experts


