Question: Using data from Table 9.2, find the force constants k for O 2 , I 2 , and N 2 . Is there a physical
Using data from Table 9.2, find the force constants k for O2, I2, and N2. Is there a physical explanation for the relative magnitudes of these values?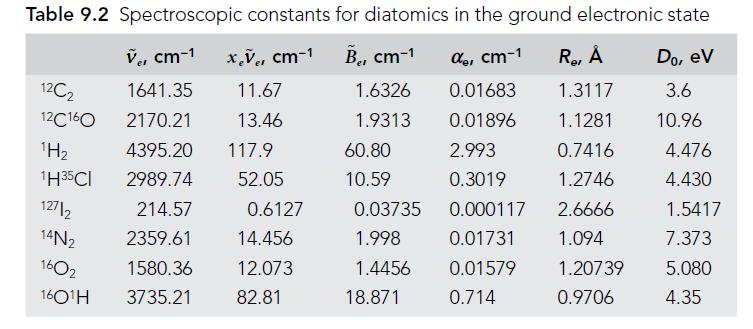
Table 9.2 Spectroscopic constants for diatomics in the ground electronic state V, cm-1 x.v, cm-1 B, cm- de, cm-1 R, Do, ev 11.67 1.6326 1.3117 3.6 13.46 1.9313 1.1281 10.96 0.7416 1.2746 2.6666 1.094 12C2 12C160 H 1H35CI 12712 14N 1602 16O1H 1641.35 2170.21 4395.20 117.9 2989.74 52.05 214.57 2359.61 1580.36 3735.21 0.6127 14.456 12.073 82.81 60.80 10.59 0.03735 1.998 1.4456 18.871 0.01683 0.01896 2.993 0.3019 0.000117 0.01731 0.01579 0.714 1.20739 0.9706 4.476 4.430 1.5417 7.373 5.080 4.35
Step by Step Solution
3.30 Rating (144 Votes )
There are 3 Steps involved in it
It seems like youve provided a table of spectroscopic constants for various diatomic molecules in their ground electronic states The constants include ... View full answer

Get step-by-step solutions from verified subject matter experts


