Consider the set of reactions and rate constants with an initial concentration of 0.75 moles of A
Question:
Consider the set of reactions and rate constants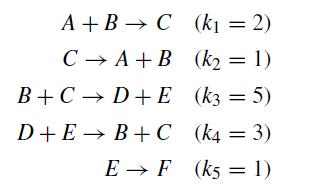
with an initial concentration of 0.75 moles of A and 1 mole of B. Write the system of ordinary differential equations describing the concentration of each species, along with appropriate initial conditions. Numerically integrate this system out to t = 10 using a stable method. Your program should automatically generate a plot of the concentrations of all species versus time. Explain the evolution of the concentrations using your knowledge of kinetics.
Step by Step Answer:
Related Book For 

Numerical Methods With Chemical Engineering Applications
ISBN: 9781107135116
1st Edition
Authors: Kevin D. Dorfman, Prodromos Daoutidis
Question Posted:




