The helium-neon laser is famous for its red-light emission at 632.8 nm. But electrons in that same
Question:
Table 13.3
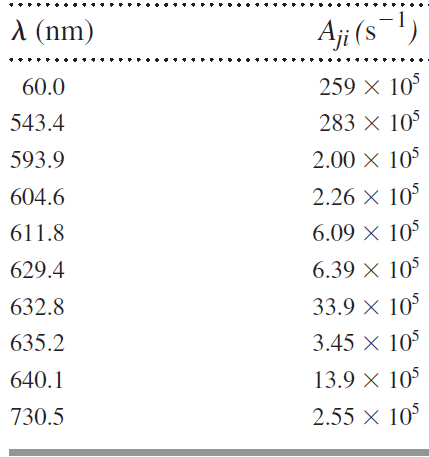
Transcribed Image Text:
A (nm) Aji (s-1) 259 × 10 60.0 283 × 10° 543.4 2.00 X 10 593.9 2.26 × 10° 604.6 6.09 × 10 611.8 6.39 × 10° 629.4 33.9 × 10 632.8 3.45 × 10 635.2 13.9 × 10 640.1 2.55 × 10° 730.5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
105 259 28...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Fusion is the process by which the sun produces energy. One experimental technique for creating controlled fusion utilizes a solid-state laser that emits a wavelength of 1060 nm and can produce a...
-
A certain helium-neon laser emits red light in a narrow band of wavelengths centered at 632.8 nm and with a "wavelength width" (such as on the scale of Figure) of 0.0100 nm. What is the corresponding...
-
A helium-neon laser emits red light at wavelength = 633 nm in a beam of diameter 3.5 mm and at an energy-emission rate of 5.0mW. A detector in the beam's path totally absorbs the beam. At what rate...
-
Modify BST to add a method rangeSearch () that takes two keys as arguments and returns an iterable over all keys that are between the two given keys. The running time should be proportional to the...
-
Gus (age 84) and Belle (age 18) are married in early 2016. Late in 2016, Belle confronts Gus about his failure to transfer to her the considerable amount of property he previously promised. Gus...
-
The Theodore has the following balances in these selected key accounts: Cash $8,000, Inventory $15,000, Prepaid Expenses $10,000, Total Current Assets of $40,000, Total Current Liabilities of...
-
Table B. 12 presents data on a heat treating process used to carburize gears. The thickness of the carburized layer is a critical factor in overall reliability of this component. The response...
-
Rex Baker and Ty Farney are forming a partnership to which Baker will devote one-half time and Farney will devote full time. They have discussed the following alternative plans for sharing income and...
-
IVANHOE COMPANY Trial Balance August 31, 2022 Before Adjustment After Adjustment Cr. Dr. Cash $10,246 Cr. Dr. $10.246 Accounts Receivable 8,272 8.836 Supplies 2,350 470 Prepaid Insurance 3,760 2,350...
-
Mary is a coworker in your agency. She has been a valuable employee to your group and one of the most respected experts in her field. You notice lately, though, that she is more reserved and is...
-
Referring to Fig. 13.6, which shows two transitions for the He-Cd laser, determine the lifetime of the higher-energy d-state. Fig. 13.6 Cadmium+ A = 1.6 10 s-1 2d3/2 353.6 nm 2p3/2 325.0 nm A = 7.8 ...
-
The beam ( = 632.8 nm) from a He-Ne laser, which is initially 3.0 mm in diameter, shines on a perpendicular wall 100 m away. Given that the system is aperture (diffraction) limited, how large is the...
-
Patents are granted for 20 years, but pharmaceutical companies can't use their patent-guaranteed monopoly powers for anywhere near this long because it takes several years to acquire approval of...
-
What is the impact of having a low number of job applicants applying for fitness instructor position because of industry competitiveness? And what will be the impact if you hire candidates with no...
-
In the United States, has the real net balance of trade been in surplus or deficit in the last 40 years? What has been the trend of the trade balance? Is the deficit greater or has it been reduced?...
-
In terms of the demand-supply model for an individual good or service, discuss the economic impact that a challenging societal factor such as the increasing mindfulness of the importance of organic...
-
You just got a new puppy, and you need to determine the best way to have him cared for while you are at work. You could enroll your puppy in a doggy daycare in your neighborhood for $22 per day....
-
Do you think that the criticism that the international economic system and its rules are skewed to favor rich countries over poor countries is correct? Please explain why or why not
-
Implement the test in Problem 8.127, and report a two-tailed p-value? Health Promotion A study looked at the influence of retirement on the level of physical activity among people ages 4564 in the...
-
Ask students to outline the reasons why the various elements of culture (social structures and control systems, language and aesthetics, religion and other belief systems, educational systems, etc.)...
-
Compute the width w(x) and velocity deficit u o (x) for the 3-dimensional turbulent wake behind a sphere.
-
A well-hit golf ball travels about 300 yards. A fast bowler or fastball pitcher throws a cricket ball or baseball at more than 90mph (miles per hour). A table-tennis player can hit a forehand return...
-
Consider an inviscid ( = 0), incompressible flow near a plane wall where a laminar boundary layer is established. Introduce coordinates x parallel to the wall and y perpendicular to it. Let the...
-
How do advanced scenario planning methodologies, such as probabilistic forecasting, sensitivity analysis, and scenario stress-testing, inform the development of robust, adaptive visions that are...
-
How can leaders sustain momentum and relevance around visionary visions amidst evolving external environments, internal dynamics, and competing priorities, fostering adaptability, resilience, and...
-
What cognitive processes underlie the formulation of visionary narratives, and how can leaders leverage storytelling techniques to evoke emotional resonance, foster buy-in, and drive transformative...

Study smarter with the SolutionInn App


