Consider the following compound: (a) Develop all reasonable retrosynthetic analyses for this compound (any diastereomer) that, at
Question:
Consider the following compound: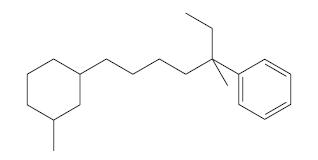
(a) Develop all reasonable retrosynthetic analyses for this compound (any diastereomer) that, at some point, involve carbon-carbon bond formation by alkylation of an alkyne ion.
(b) Write reactions, including reagents and conditions, for syntheses of this compound that correspond to the retrosynthetic analyses you developed above.
(c) Infrared spectroscopy could be used to show the presence of certain impurities in your final product that would result from leftover intermediates in your syntheses. Which of your synthetic intermediates would show IR absorptions that are distinct from those in the final product, and in what regions of the IR spectrum would the absorptions occur?
(d) Draw a three-dimensional structure for either the cis or transform of the target molecule. Use dashed and solid wedges where appropriate in the alkyl side chain and use a chair conformational structure for the ring.
Step by Step Answer:

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder





