For each of the following reactions, (1) state whether the conrotatory or disrotatory motion of the groups
Question:
For each of the following reactions, (1) state whether the conrotatory or disrotatory motion of the groups is involved, and (2) state whether you would expect the reaction to occur under the influence of heat or of light.
(a)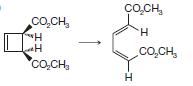
(b)
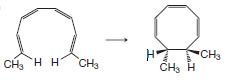
(c)
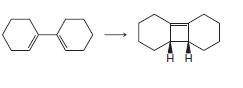
Transcribed Image Text:
CO,CH, H H CÁCH, CacH, H CO,CH, I
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
a This is conrotatory motion and since this is a 4n electron system where ...View the full answer

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
The enthalpy change for each of the following reactions was calculated using bond energies. The bond energies of XO, YO, and ZO are all equal. XX + O=O XOOX; H = 275 kJ YY + O=O YOOY; H = +275 kJ...
-
Each of the following reactions will be encountered at some point in this text. Classify each one according to whether the organic substrate is oxidized or reduced in the process. (a) (b) (c) (d) ...
-
Each of the following reactions will be encountered at some point in this text. Classify each one according to whether the organic substrate is oxidized or reduced in the process. (a) CH3C = PCH +...
-
Some ordered pairs are listed in Table 48. Table 48 Some Ordered Pairs x y -8-5 -5-3 -24 1.5 3.9 a. Construct a scatterplot by hand. b. Is there a linear association, a nonlinear association, or no...
-
Eldredge and Gould have argued that the fossil record exhibits intermittent dynamics, which they named punctuated equilibrium [169]. That adaptive evolutionary dynamics is able to generate...
-
Certain items taken from the financial statements, the notes thereto and other records of Lucky Nine Ltd have been expressed as percentages of net revenue. Required (a) By what percentage did the...
-
Entrada, an interior decorating firm, uses a job order costing system and applies overhead to jobs using a predetermined rate of $ 17 per direct labor hour. On June 1, 2013, Job # 918 was the only...
-
A person has just been appointed Immigration Czar, giving him absolute power over immigration status questions in the United States. For purposes of this exercise, U.S. immigration law is exactly...
-
John Fuji (birthdate June 6, 1981) moved from California to Washington in December 2018. His earnings and income tax withholding for 2019 for his job as a manager at a Washington apple-processing...
-
Give the stereochemistry of the product that you would expect from each of the following electrocyclic reactions.
-
Can you suggest a method for carrying out a stereospecific conversion of trans, trans-2,4-hexadiene into cis,trans-2,4-hexadiene?
-
Professor Moriarty has never taken a formal statistics course; however, he has heard about the bell-shaped curve and has some knowledge of the Empirical Rule for normal distributions. Professor...
-
3. When the Earth reaches the aphelion of 1.52 x 10 m, its speed is 2.93 x 104 m/s. After a half year, the Earth moves to the perihelion of 1.47 x 10 m. Find: (a)The speed of the Earth at the...
-
The method below takes a String parameter called str. Complete the method so that it returns a new string where each each character in the original string has been repeated 3 times. For example if...
-
Dorsey Company manufactures three products from a common input in a joint processing operation. Joint processing costs up to the split-off point total $395,000 per quarter. For financial reporting...
-
Compute the current - year income tax liability for each of the following unrelated calendar year C corporations.a . Darter Corporation has taxable income of $ 6 9 , 5 0 0 . b . Owl Corporation has...
-
Explain how greenspan was guilty of the law of small numbers What was the Fed's initial response the crisis (before Bear Stearns) What is the problem with VAR models
-
(a) Show that two diagonalizable matrices are similar if and only if they have the same eigenvalues with the same multiplicities. (b) If A is diagonalizable, show that A ~ AT. (c) Show that A ~ AT if...
-
(a) Water flows through the nozzle of a garden hose. Find an expression for m in terms of line pressure P 1 , ambient pressure P 2 , inside hose diameter D 1 , and nozzle outlet diameter D 2 . Assume...
-
A student wishes to record the UV spectrum of trans-stilbene, which has max = 308nm ( = 25,000), what concentration should be prepared if the desired absorbance is 0.5 at the maximum?
-
Indicate the types of transitions responsible for the absorptions of these compounds: Apax = 252 nm (e = 20,000) Aas - 325 nm ( = 180) a) A mux = 235 nm (e = 19,000) b) c) Amax = 299 nm ( = 20) d)...
-
Which of these compounds are expected to have an absorption maximum in the region of 200 to 400nm in their UV spectra? ) C-CH,CH, b) CH,CH,CH3 c) d) f) CH,CH,OCH,CH3 e) h) g)
-
An investor put 60 percent of his money into a risky asset offering a 10 percent return with a standard deviation of return of 8 percent, and he put the balance of his risk-free asset offering 5...
-
There is a bonus with the following characteristics: nominal value $98.650.000 annual coupon rate 4.5% term 5 years, tir 6.5% A) Calculate the price of the bond assuming it is zero coupon B)...
-
Ida Company produces a handcrafted musical Instrument called a gamelan that Is similar to a xylophone. The gamelans are sold for $949. Selected data for the company's operations last year follow:...

Study smarter with the SolutionInn App


