The first step in peptide synthesis is blocking (protection) of the amine functional group of an amino
Question:
The first step in peptide synthesis is blocking (protection) of the amine functional group of an amino acid (a compound that contains both amine and carboxylic acid functional groups). Such a reaction is shown in Section 24.7C in the reaction between Ala (alanine) and benzyl chloroformate. The functional group formed in the structure labeled Z-Ala is called a carbamate (or urethane).
(Z is a benzyloxycarbonyl group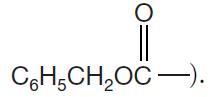
(a) Write a detailed mechanism for formation of Z-Ala from Ala and benzyl chloroformate in the presence of hydroxide.
(b) In the reaction of part (a), why does the amino group act as the nucleophile preferentially over the carboxylate anion?
(c) Another widely used amino protecting group is the 9-fluorenylmethoxycarbonyl (Fmoc) group. Fmoc is the protecting group mostvoften used in automated solid-phase peptide synthesis. Write a detailed mechanism for formation of a Fmocprotected amino acid under the conditions given in Section 24.7A.
Step by Step Answer:

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder





