Use the bond-dissociation enthalpies in Table 4-2 to calculate the heats of reaction for the two possible
Question:
Use the bond-dissociation enthalpies in Table 4-2 to calculate the heats of reaction for the two possible first propagation steps in the chlorination of isobutane. Use this information to draw a reaction-energy diagram like Figure 4-8, comparing the activation energies for formation of the two radicals.
Table 4-2
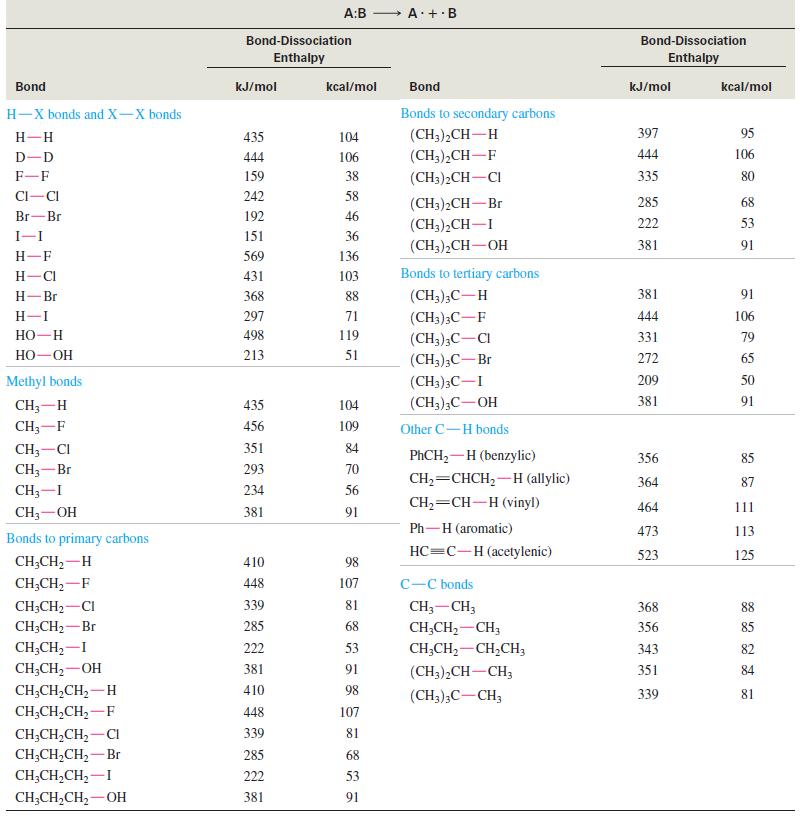
Transcribed Image Text:
A:B → A.+·B Bond-Dissociation Bond-Dissociation Enthalpy Enthalpy Bond kJ/mol kcal/mol Bond kJ/mol kcal/mol H-X bonds and X–X bonds Bonds to secondary carbons 397 (CH3),CH-H (CH3),CH-F H-H 435 104 95 D-D 444 106 444 106 F-F 159 38 (CH3)2CH-CI 335 80 Cl-CI 242 58 (CH3)2CH- Br 285 68 Br -Br 192 46 (CH3)2CH-I (CH3),CH-OH 222 53 I-I 151 36 381 91 H-F 569 136 431 103 Bonds to tertiary carbons 1D-H (CH3),C-H (CH3)3C-F (CH3);C-CI (CH3);C-Br H-Br 368 88 381 91 H-I 297 71 444 106 HO-H 498 119 331 79 НО -ОН 213 51 272 65 Methyl bonds (CH3)3C-I (CH3)3C-OH 209 50 381 91 CH3-H CH3-F 435 104 456 109 Other C-H bonds CH3-CI 351 84 PHCH,–H (benzylic) 356 85 CH3-Br 293 70 CH,— СНCH, — н (allylic) 364 87 CH;-I 234 56 CH,=CH-H (vinyl) 464 111 CH — ОН 381 91 Ph-H (aromatic) 473 113 Bonds to primary carbons НС-С- Н (асetylenic) 523 125 CH;CH2-H 410 98 CH;CH2-F 448 107 C-C bonds CH3CH2-CI 339 81 CH3-CH3 368 88 CH3CH2-Br 285 68 CH;CH,-CH; 356 85 CH;CH2-I CH;CH2-OH 222 53 CH,CH,-CH,CH3 343 82 381 91 (CH3)2CH-CH3 351 84 CH;CH,CH2-H CH;CH,CH2-F 410 98 (CH3);C-CH3 339 81 448 107 CH;CH,CH,-CI CH;CH,CH2- Br CH;CH,CH2-I 339 81 285 68 222 53 CH;CH,CH2-OH 381 91
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
In the chlorination of isobutene there are two possible first propagation steps They are ...View the full answer

Answered By

Amit Rathod
Hello, my name is Amit Rathod, I have one year of teaching experience, I solve questions in Computer Science & Mathematics Subject. I like to solve most of the difficult questions so that my subject knowledge can be increased and students can also get help.
Education: Bachelor of Engineering / Information Technology
University: Gujarat Technological University
I will complete my engineering studies in 2022.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Using bond-dissociation enthalpies from Table 4-2 (page 143), calculate the heat of reaction for each step in the free-radical bromination of methane. (b) Calculate the overall heat of reaction....
-
Carbon-carbon bond dissociation energies have been measured for alkanes. Without referring to Table 4.3, identify the alkane in each of the following pairs that has the lower carbon-carbon bond...
-
Use average bond enthalpies (Table 8.4) to estimate for the atomization of benzene, C6H6: C6H6 (g) 6C (g) + 6 H (g) Compare the value to that obtained by using Hof data given in Appendix C and...
-
Each of the following passages may be plausibly criticized by some who conclude that it contains a fallacy, but each may be defended by some who deny that the argument is fallacious. Discuss the...
-
(a) Would you need NaOH or HCl to bring the pH of 0.050 0 M HEPES (Table 8-2) to 7.45? (b) Describe how to prepare 0.250 L of 0.050 0 M HEPES, pH 7.45.
-
In a replicated 23 design (16 runs), the estimate of the model intercept is equal to one-half of the total of all 16 runs. True False
-
A capital investment project involves the purchase of a 3-D soldering station for $\$ 15,000$, producing an annual net benefit of $\$ 2,250$. The system has an 8-year useful life with a salvage value...
-
Greentea Corporation earned net income of $95,000 during the year ended December 31, 2010. On December 15, Greentea declared the annual cash dividend on its 6% preferred stock (11,000 shares with...
-
On January 1 of its first month of business, Juan in a Million, Inc., paid $48,000 for four months rent beginning in January. How much will be reported as Rent Expense on its income statement for the...
-
The St. Lucia Blood Bank, a private charity partly supported by government grants, is located on the Caribbean island of St. Lucia. The blood bank has just finished its operations for September,...
-
Write structures for a homologous series of alcohols (R-OH) having from one to six carbons.
-
Predict the ratios of products that result from chlorination of isopentane (2-methylbutane).
-
Which company shown below is likely a start-up company rather than an established company? Give reasons for your answer. Company X Company Y Cash inflow (outflow)-operating activities............$...
-
Adapting conventional web content to make it mobile friendly can require rethinking the sites information architecture to simplify navigation and revising the content. Your task: Choose the website...
-
The following exercises help you improve your knowledge of and power over English grammar, mechanics, and usage. Turn to the Handbook of Grammar, Mechanics, and Usage at the end of this book and...
-
Conduct a search on the internet and estimate what percentage of people in Germany belong to each blood group. Which type of visual would you choose to demonstrate the data? Explain your choice.
-
Businesses that need to deal with large collections of textual information can use text mining (also known as text analytics) to reduce the work involved in reading and analyzing everything from...
-
Read Figure 13.14, a solicited proposal, and (1) analyze the strengths and weaknesses of this document and (2) revise the document so that it follows this chapters guidelines. Figure 13.14: Memco...
-
When is it appropriate for companies reporting under ASPE to use the cost method to report investments?
-
Solve each equation or inequality. |6x8-4 = 0
-
The drug mechktrethamine is used in antitumor therapy. It is one of a family of compounds called nitrogen mustctrds, which also includes the antitumor drugs cyclophosphamide and chlorambucil. (a)...
-
In each of the following pairs , one. of the glycols is virtually inert to periodate oxidation. Which glycol is inefi? Explain why. CH3 CH, or
-
Provide a curved-arrow mechanism for each of the reactions in Fig. Pl1.73 that accounts for the stereochemical results. Show the structure of the unstable intermediate in each case and explain why it...
-
Given the following Python code: x = 20 while x > 10: #do something x = x 1 How many times this loop will iterate. 10 11 20 20 0
-
The HTML program is fine I want help to figure out what's wrong with CSS that would not make my website look the same as the picture I provided. @charset "UTF-8"; /* CSS Document for CA3 */ body {...
-
Consider a twisted pair link of distance 2 km. It is required to compute the amount of received power Pr, assuming the transmit power Pt = 1 Watt and the cable attenuation is 20 dB/km.

Study smarter with the SolutionInn App


