Question: Which nitrogen atom in imidazole (Problem 12.16) is more basic according to the following electrostatic potential map? Why? Problem 12.16 The five-membered heterocycle imidazole contains
Which nitrogen atom in imidazole (Problem 12.16) is more basic according to the following electrostatic potential map? Why?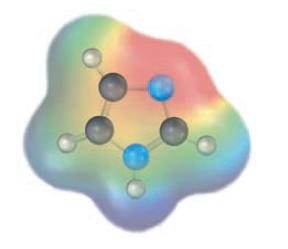
Problem 12.16
The five-membered heterocycle imidazole contains two nitrogen atoms, one “pyrrole-like” and one “pyridine-like.” Draw an orbital picture of imidazole, and indicate the orbital in which each nitrogen has its electron lone pair.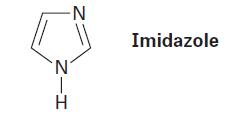
Step by Step Solution
3.34 Rating (154 Votes )
There are 3 Steps involved in it
Imidazole is a fivemembered heterocycle with two nitrogen atoms these atoms are not equivalent The n... View full answer

Get step-by-step solutions from verified subject matter experts


