(a) Explain why the following equilibrium lies far to the right. (b) Chemists had always assumed that...
Question:
(a) Explain why the following equilibrium lies far to the right.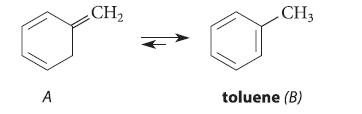
(b) Chemists had always assumed that this reaction would be so fast that compound A could never be isolated. However, this compound was prepared in 1962 and shown to be stable in the gas phase at 70°C, despite the favorable equilibrium constant for its transformation to B. Show why the conversion of A into B above would not be expected to occur as a concerted reaction.
(c) Would you expect a concerted mechanism for the following reaction to be equally slow? Why?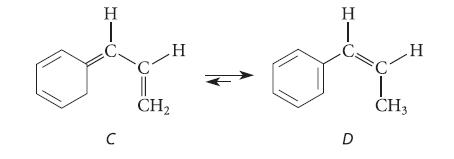
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





