A scientist at an Air Force Research Laboratory in California has studied highly energetic materials (explosivematerials) and,
Question:
A scientist at an Air Force Research Laboratory in California has studied “highly energetic materials” (explosive materials) and, more significantly, has lived to tell about it. In 2000, he and his equally adventurous collaborators determined the X-ray crystal structure of the N5+ cation; most salts of this cation are highly explosive. This species is the first ever isolated in modern times that contains more than three contiguously bonded nitrogens. The crystal structure revealed a “V-shape” for the cation, as follows: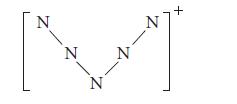
Notice that the lines do not indicate the bonding pattern, but only the shape.
(a) Draw an acceptable Lewis structure, including unshared electron pairs, that accounts for the shape of the molecule and its overall plus charge.
Explain why your molecule meets these criteria. Be sure to show in your structure the formal charge of every atom with nonzero formal charge.
(b) Using the curved-arrow notation, derive two additional resonance structures for this cation that meet the same criteria.
Step by Step Answer:






