(a) The dipole moment of acetaldehyde is 2.7 D and that of propene is 0.5 D. Even...
Question:
(a) The dipole moment of acetaldehyde is 2.7 D and that of propene is 0.5 D. Even though they have about the same molecular mass, they differ in boiling point by about 68°C (–47°C and 121°C). Which has the higher boiling point? Why?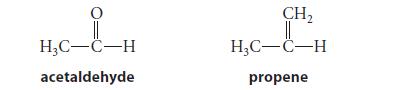
(b) Given that alkenes and alkanes with the same branching pattern and number of carbons have about the same boiling points, show where you would expect the curve for aldehyde boiling points to fall in Fig. 8.2b. Explain.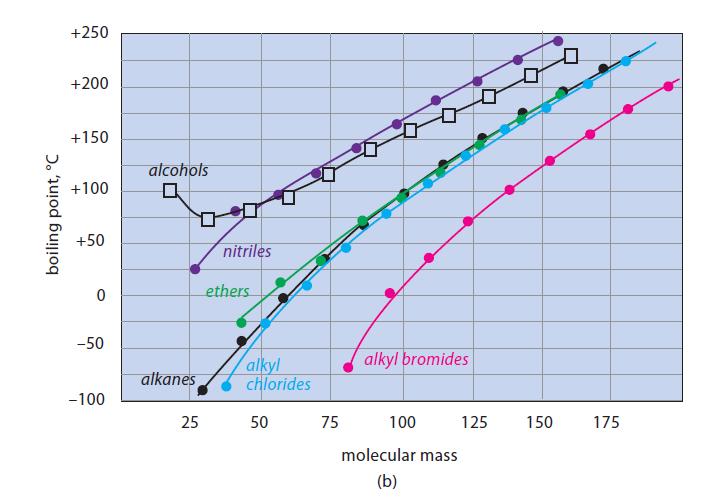
Transcribed Image Text:
H₂C-C-H acetaldehyde CH₂ || H₂C-C-H propene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a Acetaldehyde has a higher boiling point 21C than propene 47C because there are stronger ...View the full answer

Answered By

Gauri Hendre
I worked as EI educator for Eduphy India YT channel. I gave online tutorials to the students who were living in the villages and wanted to study much more and were preparing for NEET, TET. I gave tutions for topics in Biotechnology. I am currently working as a tutor on course hero for the biochemistry, microbiology, biology, cell biology, genetics subjects. I worked as a project intern in BAIF where did analysis on diseases mainly genetic disorders in the bovine. I worked as a trainee in serum institute of India and Vasantdada sugar institute. I am working as a writer on Quora partner program from 2019. I writing on the topics on social health issues including current COVID-19 pandemic, different concepts in science discipline. I learned foreign languages such as german and french upto A1 level. I attended different conferences in the science discipline and did trainings in cognitive skills and personality development skills from Lila Poonawalla foundation. I have been the member of Lila poonawalla foundation since 2017. Even I acquired the skills like Excel spreadsheet, MS Office, MS Powerpoint and Data entry.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
1. How important is it to have a mix of products? When shoes slack off during the winter, the apparel business picks up. What are the implications of seasonal products? What problems does this create...
-
If the WACC of a company is less than the Terminal Growth Rate how do you calculate Present Terminal Value of FFCF (Free Firm Cash Flow) for a Discounted Cash Flow Model? I currently have a WACC of...
-
Which description does NOT fit in description of "issues" in the context of international standards? a. An unsettled matter. b. A vital matter. c. A change in the environment. d. A concern or...
-
ETHICS: Hector works in Zoeys importing firm. Zoey overhears Hector on the phone say, OK, 30,000 ski parkas at $80 per parka. Youve got yourself a deal. Thanks a lot. When Hector hangs up, Zoey is...
-
Briefly state Adam Smith's four requirements for a good tax system.
-
In general how have interest rates changed since the late 1980s according to Figure 11.1? FIGURE 11.1 Interest and Inflation Rates, 1988-2010 10p Rate (%) 8 6 10-Year Treasury Bonds 4 2 AAA Corporate...
-
A blood bank wants to determine the least expensive way to transport available blood donations from Pittsburg and Staunton to hospitals in Charleston, Roanoke, Richmond, Norfolk, and Suffolk. Figure...
-
A manager could increase his or her accounting income-based bonus by Multiple choice question. delaying discretionary expenses. delaying shipments of its products. disclosing additional contingent...
-
The boiling points of the 1,2-dichloroethylene stereoisomers are 47.4C and 60.3C. Which stereoisomer has each boiling point? Explain.
-
From the data in Fig. 8.1, tell which bonds have the greater amount of p character (Secs. 1.9B and 6.9B): CO bonds or CS bonds. Explain. Fig. 8.1 1.426 HC HC 109 0.96 H 1.413 A 111.4 CH3 1.82 A...
-
Temple Industrial's production cost report for its Packaging Department reveals that the cost per equivalent unit started and completed in November was $165. The same report reveals that the cost per...
-
1. Create SMART marketing objectives for a roasting coffee business. 2. Describe tactics, KPIs per tactic, and targets per tactic for a roasting coffee business.
-
What would be the criteria for the alternatives? What are the alternatives for Mike? What are the recommendations, implementations and monitoring and control for this scenario? How can the...
-
comple this Risk management plan table Company : Deebees Organics Target Country: Finland Rank Name of Risk Score (I*L) Rationale Plan Strategy 1 Competitors Risk 5,5 = 25 2 Aging Population Risk...
-
What were Autodesk's objectives for the project? How was IPD defined for this project? In particular, what do you think about its risk management model, joint management structure, and compensation...
-
You are newly hired to manage an technology informationteam in a company wishing to develop a new employee scheduling system. Your predecessor had already analyzed the functional needs that the...
-
Learning how to use software takes time. So once customers have learned to use a particular software package, it is easier to sell them software upgrades than to convince them to switch to new...
-
An interest bearing promissory note for 90 days at 5.6% p.a. has a face value of $120,000. If the note is discounted 20 days after the issue date at a rate of 6.8% p.a., calculate the amount of...
-
Show the molecular ions formed from these compounds: b) a) CH,NHCH,
-
Show equations for the major fragmentations you would expect from the molecular ions of these compounds. List the m/z of the productions. CH3 CH2 a) CH;CH-CH,CH-CH; b) CH;CH,CHCH2CH3
-
(a) The base ion in the mass spectrum of 3-ethyl-2-methylpentane occurs at m/z 43m; show the fragmentation that produces this ion. (b) What other fragment would you predict to provide a major peak in...
-
5. (10 points) The quasar 3C273 has a luminosity of 2 x 1039 W. Assuming that 10% of the mass that falls onto the accretion disk is converted into luminosity, how fast is 3C273 accreting gas? Give...
-
How much energy can be obtained from the U235 in 1 kilogram of natural uranium? Keep in mind that in nature U235 only makes up 0.7% of the total sample. How many grams of the 1 kg sample are...
-
An accretion disk around a black hole consists of particles in approximately circular orbits about a central mass. The solar system is made up of planets ( made of particles ) in orbit about a...

Study smarter with the SolutionInn App


