(a) Use the relative bond lengths of the CC and CO bonds to predict which of the...
Question:
(a) Use the relative bond lengths of the C—C and C—O bonds to predict which of the following two equilibria lies farther to the right. (That is, predict which of the two compounds contains more of the conformation with the axial methyl group.)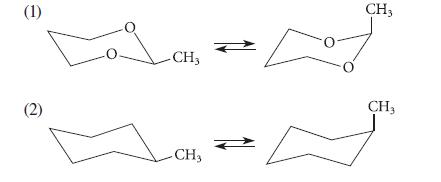
(b) Which one of the following compounds contains the greater amount of gauche conformation for internal rotation about the bond shown? Explain.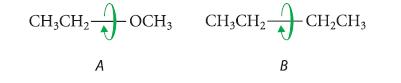
Transcribed Image Text:
(1) (2) -CH3 - CH3 ← CH3 CH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Equilibrium 2 contains more of the conformation with the methyl group in an axial position than eq...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
a. Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (CO23-) b. What would you expect the charge to be on each oxygen atom?
-
Kenny operates a store, where he sells feed and other supplies to farmers. Heather purchases a $20,000 tractor from Kenny and pays Kenny with $18,000 in cash and $2,000 in corn. How much gross income...
-
Name five techniques you can use to ensure that visual aids do not distort graphic information.
-
Leon exchanges an office building which he held as investment property for a bowling alley. His office building has a basis of $175,000 and a fair market value of $160,000, and it is subject to a...
-
Problem 8 is the same as problem 7 with respect to initial measurement of the ARO liability. Now assume that Tadpoles credit standing improves over time, causing the credit-adjusted risk-free rate to...
-
Annual Adjustments Palmer Industries prepares annual financial statements and adjusts its accounts only at the end of the year. The following information is available for the year ended December 31,...
-
6. For the following code fragment, what are the values of the array elements after the statements are executed? int[] T = {2, 2, 2, 3, 3, 3}; for (int i = 1; i < T.length; i++) { T[i] = (T[i-1] +...
-
Consider the reaction analyzed in Study Problem 3.2 (p. 91), reproduced below. Identify the nucleophilic center, the electrophilic center, and the leaving group in the forward direction. (Dont...
-
Offer an explanation for each of the following observations. (a) Compound A exists mostly in a chair conformation with an equatorial OH group, but compound B prefers a chair conformation with an...
-
Establish identity. cot(20) tan 0) 5(cot 0
-
Iramba Sea Foods Investment is negotiating a Loan from Tanzania Investments Bank which has kindly requested the Company to prepare and submit their cash budget for the first half year. The following...
-
Dilemmas As a purchasing manager, you may be faced with making product decisions that conflict with your own strongly-held values and beliefs. For example: You are concerned about the environment,...
-
What is the relationship between communication and health inequality? Specify the pathways through which communication influences health inequality.
-
2. The Quickie Mart receives a shipment of batteries on Jan 20th. 15 cases are delivered. The price is $45 per case with discounts of 6% and 5% and payment terms of 1/15, n/30. a. What is the last...
-
Sarah Green has been an accountant for 12 years. She worked in a small medical practice for two years after earning her BS in Accounting and then chose to work freelance for five years. For the past...
-
Here is the deal: You can pay your college tuition at the beginning of the academic year or the same amount at the end of the academic year. You either already have the money in an interest-bearing...
-
Carlton Stokes owns and operates a car-detailing business named SuperShine & Detailing. For $150, Carltons business will hand wash and wax customers cars, vacuum the interior, and thoroughly clean...
-
This alkynes hydration reaction can occur without added Hg2+. Show all the steps in themechanism. H,SO, PhC=CH + H20 PHCCH3
-
Explain which compound has a faster rate of reaction withHCI: b) or or NO2 or
-
The addition of C1 to (E)-2-pentene produces a recemic mixture of (2R,3S)-2,3- dichloropentane and its enantiomers, 2R,3S)-2,3- dichloropentane. (a) Show the structure of the two chloronium ions that...
-
The sugar tax is an important policy decision by the government to discourage its citizens from consuming sugar. Using the principles of evaluating a tax, discuss whether or not the sugar tax is good
-
For the following demand equations calculate the coefficient for price elasticity of demand when P=10. (a) Q =100????0.01???? (b) Q = 100-2lnP (c) Q = - ???????????? + 10
-
In February 2023, the Mpumalanga province in South Africa experienced floods that have devastated many establishments across the Crocodile River. Assuming the Mpumalanga Provincial Treasury has...

Study smarter with the SolutionInn App


