An optically active compound A has the formula C 8 H 13 Br. Compound A gives no
Question:
An optically active compound A has the formula C8H13Br. Compound A gives no reaction with Br2 in CH2Cl2, but it reacts with K+(CH3)3C—O– to give a single new compound B in good yield. Compound B decolorizes Br2 in CH2Cl2 and takes up hydrogen over a catalyst. When compound B is treated with ozone followed by aqueous H2O2, dicarboxylic acid C is isolated in excellent yield; notice its cis stereochemistry.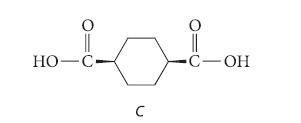
Identify compounds A and B, and account for all observations.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





