Around 1900, Moses Gomberg, a pioneer in freeradical chemistry, prepared the triphenylmethyl radical, Ph 3 C?, sometimes
Question:
Around 1900, Moses Gomberg, a pioneer in freeradical chemistry, prepared the triphenylmethyl radical, Ph3C?, sometimes called the trityl radical (trityl 5 triphenylmethyl).
(a) Explain why the trityl radical is an unusually stable radical.
(b) The trityl radical is known to exist in equilibrium with a dimer that, for many years, was assumed to be hexaphenylethane, Ph3C—CPh3. Show how hexaphenylethane could be formed from the trityl radical.
(c) In 1968 the structure of this dimer was investigated using modern methods and found not to be hexaphenylethane, but rather the following compound. Using the fishhook notation, show how this compound is formed from two trityl radicals, and explain why this compound is formed instead of hexaphenylethane.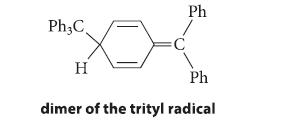
Step by Step Answer:






