Draw the structure of the major form of each of the following compounds present in an aqueous
Question:
Draw the structure of the major form of each of the following compounds present in an aqueous solution containing initially one molar equivalent of 1 M HCl. Explain your reasoning.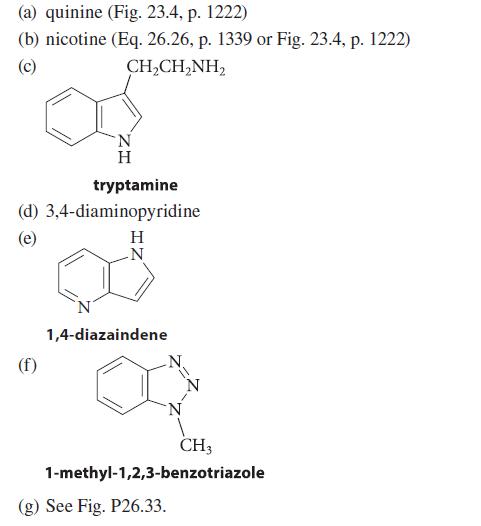
Transcribed Image Text:
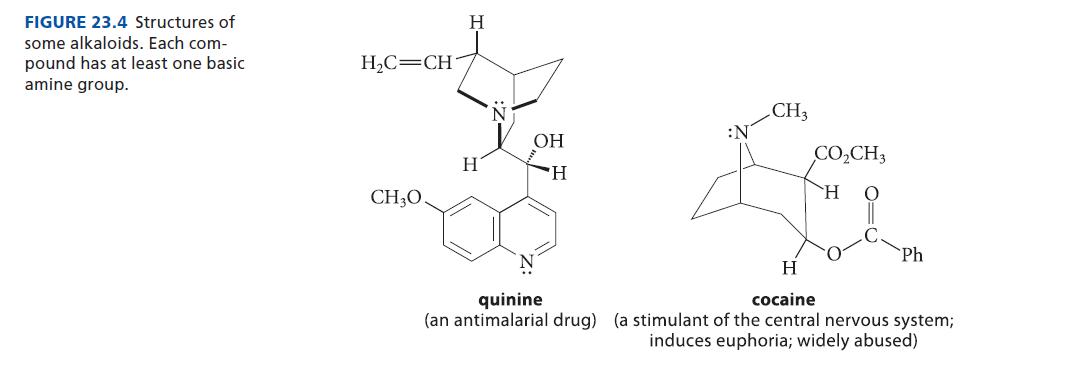
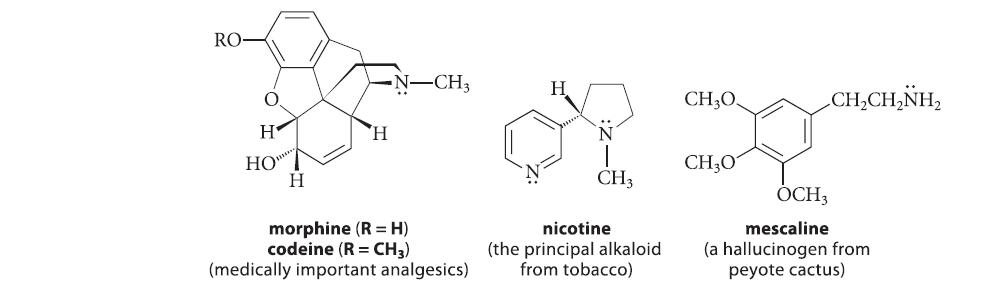
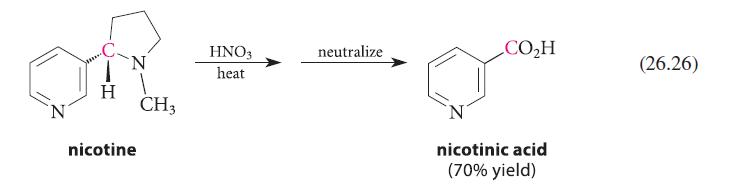
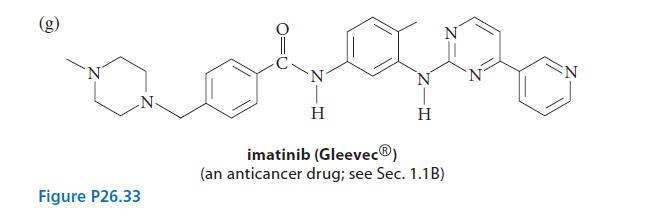
(a) quinine (Fig. 23.4, p. 1222) (b) nicotine (Eq. 26.26, p. 1339 or Fig. 23.4, p. 1222) (c) CH,CH,NH, (d) (e) H tryptamine 3,4-diaminopyridine H N 1,4-diazaindene N (g) See Fig. P26.33. CH3 1-methyl-1,2,3-benzotriazole
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a b Because alkylamines are more basic than pyridines or quinolines the conjugate acid of quinine is ...View the full answer

Answered By

Robert Mwendwa Nzinga
I am a professional accountant with diverse skills in different fields. I am a great academic writer and article writer. I also possess skills in website development and app development. I have over the years amassed skills in project writing, business planning, human resource administration and tutoring in all business related courses.
4.90+
187+ Reviews
378+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What are the reasons that electric car drivers lease their cars If you were going to acquire an electric or hybrid vehicle, would you lease or purchase Explain your reasoning
-
An aqueous solution containing MgCl2 and HCl was analyzed by first titrating a 25.00-mL aliquot to a bromocresol green end point with 17.53 mL of 0.02932 M NaOH. A 10.00-mL aliquot was then diluted...
-
In an aqueous solution containing sodium bicarbonate, aniline reacts quickly with bromine to give 2, 4, 6-tribromoaniline. Nitration of aniline requires very strong conditions, however, and the...
-
Goods 1 and 2 are available at dollar prices of p1 per unit of Good 1 and p2 per unit of Good 2. A utility function U(x 1 , x 2 ) is a function representing the utility or benefit of consuming xj...
-
Rachael Ray Corporation had the following transactions. 1. Sold land (cost $12,000) for $15,000 2. Issued common stock for $20,000 3. Recorded depreciation of $17,000 4. Paid salaries of $9,000 5....
-
Zion Manufacturing had always made its components in-house. However, Bryce Component Works had recently offered to supply one component, K2, at a price of $25 each. Zion uses 10,000 units of...
-
A spaceship flies past an experimenter who measures its length to be one-half the length he had measured when the spaceship was at rest. An astronaut aboard the spaceship notes that his clock ticks...
-
Angina, Inc., has 5 million shares outstanding. The firm is considering issuing an additional 1 million shares. After selling these shares at their $20 per share offering price and netting 95% of the...
-
Prepare common size balance sheets and income statements for the three-year period and discuss your observations. 2. Compute the ratios listed in Exhibit 3 for Anandam for the last two years over the...
-
The following compound is a very strong base; its conjugate acid has a pK a of about 13.5. Give the structure of its conjugate acid and show that it is stabilized by resonance. CH3 -Z:
-
(a) Carry out an orbital symmetry analysis to show that suprafacial [1,5] carbon migrations should occur with retention of configuration in the migrating group. (b) Indicate what type of sigmatropic...
-
In Exercises solve the Bernoulli differential equation. The Bernoulli equation is a well-known nonlinear equation of the form that can be reduced to a linear form by a substitution. The general...
-
What is an annuity, and what features determine the amount of income associated with such a contract?
-
Are gains and losses from the sale or exchange of personal use assets recognized for tax purposes?
-
What factors tend to indicate that excess accumulations exist?
-
Differentiate between the following: active income, passive income, and portfolio income.
-
If a corporation begins business on June 12, 2019, when may it close its first tax year?
-
Can the annual new orders for manufacturing in the United States be predicted by the raw steel production in the United States? Shown on the next page are the annual new orders for 10 years according...
-
For a Poisson process of rate , the Bernoulli arrival approximation assumes that in any very small interval of length , there is either 0 arrivals with probability 1- or 1 arrival with probability ....
-
In each compound, identify (1) the diastereotopic fluorines, (2) the enantiotopic fluorines, (3) the homotopic fluorines, and (4) the constitutionally nonequirnalent fluorines. CH,
-
(a) When propanol containing deuterium (D, or 2H; rather than hydrogen at the oxyger, CH3CH2CH2OD, is treated with an excess of H2O containing a catalytic amount of NaOH, 1-propanol is formed...
-
Indicate whether each of the following transformations is an oxidation, a reduction, or neither, and how many electrons are involved in each oxidation or reduction process. (a) (b) OCHs + CH OH CH3...
-
would all the income be taxed under the other taxes rates chart or just the qualified income? describe detail.
-
Analyze how Crazy Eddie's fictitious purchase discounts affected the company's profit on the income statement. Construct example of the effect on the income statement. Examine how the premature...
-
Assume the following facts regarding Granite Corporation and Alex Baldwin, one of its shareholders: $0 Granite Corporation's Accumulated E&P $2,000,000 Granite Corporation's Current E&P 4,000 Total...

Study smarter with the SolutionInn App


