Enols, like phenols, have pK a values of ~1011. However, the pK a of warfarin, a widely
Question:
Enols, like phenols, have pKa values of ~10–11. However, the pKa of warfarin, a widely used anticoagulant, is 5.0.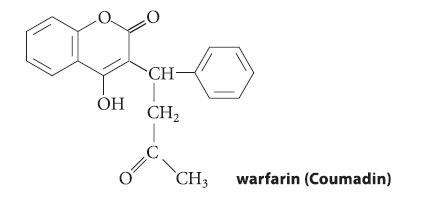
(a) Give the structure of the conjugate base of warfarin.
(b) Use resonance arguments to justify the unusually low pKa of warfarin.
(c) What is the predominant form of warfarin—enol or conjugate base—at physiological pH (7.4)?
Transcribed Image Text:
ОН 0 CH- CH₂ CH3 warfarin (Coumadin)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a b The resonance structures below show that the negativ...View the full answer

Answered By

Nicholas Maina
Throughout my tutoring journey, I've amassed a wealth of hands-on experience and honed a diverse set of skills that enable me to guide students towards mastering complex subjects. My proficiency as a tutor rests on several key pillars:
1. Subject Mastery:
With a comprehensive understanding of a wide range of subjects spanning mathematics, science, humanities, and more, I can adeptly explain intricate concepts and break them down into digestible chunks. My proficiency extends to offering real-world applications, ensuring students grasp the practical relevance of their studies.
2. Individualized Guidance:
Recognizing that every student learns differently, I tailor my approach to accommodate various learning styles and paces. Through personalized interactions, I identify a student's strengths and areas for improvement, allowing me to craft targeted lessons that foster a deeper understanding of the material.
3. Problem-Solving Facilitation:
I excel in guiding students through problem-solving processes and encouraging critical thinking and analytical skills. By walking learners through step-by-step solutions and addressing their questions in a coherent manner, I empower them to approach challenges with confidence.
4. Effective Communication:
My tutoring proficiency is founded on clear and concise communication. I have the ability to convey complex ideas in an accessible manner, fostering a strong student-tutor rapport that encourages open dialogue and fruitful discussions.
5. Adaptability and Patience:
Tutoring is a dynamic process, and I have cultivated adaptability and patience to cater to evolving learning needs. I remain patient through difficulties, adjusting my teaching methods as necessary to ensure that students overcome obstacles and achieve their goals.
6. Interactive Learning:
Interactive learning lies at the heart of my approach. By engaging students in discussions, brainstorming sessions, and interactive exercises, I foster a stimulating learning environment that encourages active participation and long-term retention.
7. Continuous Improvement:
My dedication to being an effective tutor is a journey of continuous improvement. I regularly seek feedback and stay updated on educational methodologies, integrating new insights to refine my tutoring techniques and provide an even more enriching learning experience.
In essence, my hands-on experience as a tutor equips me with the tools to facilitate comprehensive understanding, critical thinking, and academic success. I am committed to helping students realize their full potential and fostering a passion for lifelong learning.
4.90+
5+ Reviews
16+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The tooth numbers for the gear train illustrated are N2 = 20, N3 = 16, N4 = 30, N6 = 36, and N7 = 46. Gear 7 is fixed. If shaft a is turned through 10 revolutions, how many turns will shaft bmake? 6...
-
Students frequently perform the following type of calculation to introduce a zero into a matrix: However, 3R2 - 2R1 is not an elementary row operation. Why not? Show how to achieve the same result...
-
Predict the result of each multiplication by a diagonal matrix, and then check by multiplying it out. (a) (b) 3 0 1 2 0 3 4 4 1 2 0 2) 3 4
-
Six months ago, Qualitybank issued a $ 100 million, one-year-maturity CD, denominated in British pounds (Euro CD). On the same date, $ 60 million was invested in a -denominated loan and $ 40 million...
-
You have been requested by a friend named Dean McChesney to advise him on the effects that certain transactions will have on his business. Time is short, so you cannot journalize the transactions....
-
In an NBC News/Wall Street Journal poll of 1,000 American adults conducted August 59, 2010, 44% of respondents approved of the job that Barack Obama was doing in handling the economy. a. Compute the...
-
If a well-behaved investment alternative's internal rate of return (IRR) is equal to MARR, which of the following statements about the other measures of worth for this alternative must be true? 1....
-
Beginning and Ending Fund Balances. The following information is provided about the Village of Wymettes General Fund operating statement and budgetary accounts for the fiscal year ended June 30,...
-
Suzanne Michaels from Howie's Pizza was asked to calculate the break-even point for a new line of gourmet pizzas (prebaked, ready to take home). The selling price will be $25 per pizza. The labor...
-
Identify compounds A, B, and C from the following information. (a) Compound A, C 8 H 10 O, is insoluble in water but soluble in aqueous NaOH solution, and yields 3,5-dimethylcyclohexanol when...
-
Although enols are unstable compounds (Sec. 14.5A), suppose that the acidity of an enol could be measured. Which would be more acidic: enol A or alcohol B? Why? OH OH I H3C-C=CH H3C-CH-CH3 A B
-
The function vdist \((x 0,1)\) returns a list of linguistic distances from the designated point \(\mathrm{x} 0\) in linguistic region 1 to each of the 504 language locations. Show that Atkinson's...
-
Discuss the topic - Local, regional and global development issues. 2. Discuss some of the development issues faced on the regional and global scene. 3.. Name and describe the functions of some of the...
-
Explain why? globalization on communication could possible lead to mash or mash up of civilization where in certain customs of a person or place would combine and amalgamate with one another...
-
Identify your choice of the top five elements of a human resources strategy that link to an organization's overall strategic goals. Support your selections using this week's resources. Also, support...
-
Prepare to communicate complex information and negotiate outcomes. What information (related to your selected workplace project or issue) must be conveyed and negotiated? With whom will the...
-
complex and sophisticated technologies have created problems for society because they have outstripped the human ability to control them; the naked ape is not able to act responsibly with the...
-
Sweet Tooth Confectionary incurred $157,000 of manufacturing overhead costs during the year just ended. However, only $141,000 of overhead was applied to production. At the conclusion of the year,...
-
Solve the relation Exz:Solve therelation ne %3D
-
Show how you might prepare each of the following amines through reductive amination: (a) (b) (c) NH2 CH3 CH3
-
When phenyl isothiocyanate, C6H5N == C == S, is reduced with lithium aluminum hydride, the product formed has these spectral data: MS (m/z): 107, 106 IR (cm-1): 3330 (sharp), 3050, 2815, 760, 700 1H...
-
When N,N'-diphenylurea (A) is reacted with tosyl chloride in pyridine, it yields product B. The spectral data for B include: MS (m/z): 194 (M+.) IR (cm-1): 3060, 2130, 1590, 1490, 760, 700 1H NMR...
-
Homework 2: Answer the following questions Q: 1. Show the ode(t) regarding Y=GF for sys. A. 2. Show the ode(t) regarding Y=feedback(K*G,1)*F for sys. B. 3. Show the ode(t) regarding...
-
Part B: Process Planning To unravel the mysteries of the capabilities of this refrigeration valve, your team embark on a quest to create a wondrous prototype using machining processes! You summon the...
-
Question 6. A vehicle is being used for a towing event with the gear train shown in Figure 3. The vehicle's engine is designed to operate under the following conditions: 400 Nm of output torque at...

Study smarter with the SolutionInn App


